Stria terminalis
| Stria terminalis | |
|---|---|
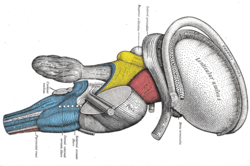 Dissection of brain-stem. Lateral view. (Stria terminalis labeled at upper right.) | |
 Bed nucleus of the stria terminalis of the mouse brain | |
| Details | |
| Identifiers | |
| Latin | stria terminalis |
| NeuroNames | 286 |
| NeuroLex ID | birnlex_937 |
| TA98 | A14.1.09.275 |
| TA2 | 5592 |
| FMA | 61974 |
| Anatomical terms of neuroanatomy | |
The stria terminalis (or terminal stria) is a structure in the brain consisting of a band of fibers running along the lateral margin of the ventricular surface of the thalamus. Serving as a major output pathway of the amygdala, the stria terminalis runs from its centromedial division to the ventromedial nucleus of the hypothalamus.
Anatomy[]
The stria terminalis covers the superior thalamostriate vein, marking a line of separation between the thalamus and the caudate nucleus as seen upon gross dissection of the ventricles of the brain, viewed from the superior aspect.
The stria terminalis extends from the region of the interventricular foramina to the temporal horn of the lateral ventricle, carrying fibers from the amygdala to the septal nuclei, hypothalamic, and thalamic areas of the brain. It also carries fibers projecting from these areas back to the amygdala.
Bed nucleus of the stria terminalis (BNST)[]
The activity of the bed nucleus of the stria terminalis correlates with anxiety in response to threat monitoring.[1] It is thought to act as a relay site within the hypothalamic-pituitary-adrenal axis and regulate its activity in response to acute stress.[2] However, the stress response is time related and the BNST does not activate for contextual fear. This means that a sudden scary situation that is under ten minutes long, does not activate the BNST.[3] It is also thought to promote behavioral inhibition in response to unfamiliar individuals, by input from the orbitofrontal cortex.[4] Bilateral disruption of this pathway has been shown to attenuate reinstatement of drug seeking behaviour in rodents.[5]
This nucleus is known to project inhibitory fibers to the lateral hypothalamus and participate in the control of feeding in rodents. Optogenetic activation of this inhibitory pathway rapidly produced voracious feeding behavior in well-fed mice and optogenetic inhibition of this pathway reduces food intake even in starved animals.[6]
Sexual dimorphism[]
The central subdivision of the bed nucleus of the stria terminalis (BSTc) is sexually dimorphic. On average, the BSTc is twice as large in men as in women and contains twice the number of somatostatin neurons.[7] A sample of six post-mortem, long-term hormone replacement therapy (HRT) treated trans women (male-to-female) were found to have a female-typical number of cells in the BSTc, whereas a trans man (female-to-male) was found to have a male-typical number.[8][9] The authors (Jiang-Ning Zhou, Frank PM Kruijver, Dick Swaab) also examined subjects with hormone-related disorders and found no pattern between those disorders and the BSTc while the single untreated male-to-female transsexual had a female-typical number of cells. They concluded that the BSTc provides evidence for a neurobiological basis of gender identity and proposed that such was determined before birth.
Hormone replacement therapy has been shown to influence hypothalamic size,[10] even though the study tried to do this by including non-transsexual male and female controls which, for a variety of medical reasons, had experienced hormone reversal.[8] The statement about the neurobiological basis from birth has later been brought to question, though not refuted, by a follow up study by the same group which found that the sexual dimorphism of the BSTc is not present before adulthood (approximately 22 years of age) even though transsexuals report being aware of their gender identity since childhood.[11]
Since somatostatin-expressing neurons typically block dendritic inputs to the postsynaptic neuron, thus inhibiting signals traveling through associated structures, it is believed that the larger bed nucleus of the stria terminalis found in men (including transgender men) reduce the startle response in men and may be responsible for the higher incidence of specific phobias in women, and a possible source for the stereotype of women being afraid of mice.[12]
Oxytocin receptor activity in the BNST is important for social recognition in rats. Both male and female rats that received a microinjection of oxytocin receptor antagonist had lower social recognition scores than rats that received a vehicle injection, and microinjections of oxytocin into the BNST enhanced social memory in male, but not female, rats.[13]
Reduction of the size of the bed nucleus of the stria terminalis has been observed in pedophilic male perpetrators, in addition to reductions in the right amygdala, hypothalamus and abnormalities in related structures. The authors propose that childhood deficits in the BNST and medial amygdala may cause inhibition of sexual maturity.[14]
References[]
- ^ Somerville L, Whalen P, Kelley W (2010). "Human Bed Nucleus of the Stria Terminalis Indexes Hypervigilant Threat Monitoring". Biol Psychiatry. 68 (5): 416–424. doi:10.1016/j.biopsych.2010.04.002. PMC 2921460. PMID 20497902.
- ^ Choi D, Furay A, Evanson N, Ostrander M, Ulrich-Lai Y, Herman J (2007). "Bed Nucleus of the Stria Terminalis Subregions Differentially Regulate Hypothalamic–Pituitary–Adrenal Axis Activity: Implications for the Integration of Limbic Inputs". J Neurosci. 27 (8): 2025–34. doi:10.1523/JNEUROSCI.4301-06.2007. PMC 6673539. PMID 17314298.
- ^ Hammack SE, Todd TP, Kocho-Schellenberg M, Bouton ME (2015). "Role of the Bed Nucleus of the Stria Terminalis in the Acquisition of Contextual Fear at Long or Short Context-Shock Intervals". Behavioral Neuroscience. 129 (5): 673–678. doi:10.1037/bne0000088. PMC 4586907. PMID 26348716.
- ^ Fox A, Shelton S, Oakes T, Converse A, DavidsonR, Kalin N (2010). "Orbitofrontal Cortex Lesions Alter Anxiety-Related Activity in the Primate Bed Nucleus of Stria Terminalis". J Neurosci. 30 (20): 7023–27. doi:10.1523/JNEUROSCI.5952-09.2010. PMC 2915894. PMID 20484644.CS1 maint: multiple names: authors list (link)
- ^ Suzanne Erb · Natalina Salmaso · Demetra Rodaros Jane Stewart (2001). "A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats". Psychopharmacology. 158 (4): 360–65. doi:10.1007/s002130000642. PMID 11797056. S2CID 23284158.
- ^ Jennings, Joshua H.; Rizzi, Giorgio; Stamatakis, Alice M.; Ung, Randall L.; Stuber, Garret D. (2013-09-27). "The Inhibitory Circuit Architecture of the Lateral Hypothalamus Orchestrates Feeding". Science. 341 (6153): 1517–1521. doi:10.1126/science.1241812. ISSN 0036-8075. PMC 4131546. PMID 24072922.
- ^ Swaab D (2007). "Sexual differentiation of the brain and behavior". Best Pract Res Clin Endocrinol Metab. 21 (3): 431–44. doi:10.1016/j.beem.2007.04.003. PMID 17875490.
- ^ Jump up to: a b Zhou J, Hofman M, Gooren L, Swaab D (1995). "A sex difference in the human brain and its relation to transsexuality". Nature. 378 (6552): 68–70. Bibcode:1995Natur.378...68Z. doi:10.1038/378068a0. hdl:20.500.11755/9da6a0a1-f622-44f3-ac4f-fec297a7c6c2. PMID 7477289. S2CID 4344570.
- ^ Kruijver F, Zhou J, Pool C, Hofman M, Gooren L, Swaab D (2000). "Male-to-female transsexuals have female neuron numbers in a limbic nucleus". J. Clin. Endocrinol. Metab. 85 (5): 2034–41. doi:10.1210/jcem.85.5.6564. PMID 10843193.
- ^ Hulshoff Pol HE, Cohen-Kettenis PT, Van Haren NE, Peper JS, Brans RG, Cahn W, et al. (2006). "Changing your sex changes your brain: Influences of testosterone and estrogen on adult human brain structure". European Journal of Endocrinology. 155 (Suppl. 1): S107–S114. doi:10.1530/eje.1.02248.
- ^ Chung W, De Vries G, Swaab D (2002). "Sexual differentiation of the bed nucleus of the stria terminalis in humans may extend into adulthood". J Neurosci. 22 (3): 1027–33. doi:10.1523/JNEUROSCI.22-03-01027.2002. PMC 6758506. PMID 11826131.
- ^ Cameron, Alasdair (2004). Crash Course Psychiatry. Elsevier Ltd. ISBN 978-0-7234-3340-8.
- ^ Dumais, KM; Alonso, AG; Immormino, MA; Bredewold, R; Veenema, AH (February 2016). "Involvement of the oxytocin system in the bed nucleus of the stria terminalis in the sex-specific regulation of social recognition". Psychoneuroendocrinology. 64: 79–88. doi:10.1016/j.psyneuen.2015.11.007. PMC 4698213. PMID 26630388.
- ^ Schiltz K, Witzel J, Northoff G, Zierhut K, Gubka U, Fellman H, Kaufmann J, Tempelmann C, Wiebking C, Bogerts B (2007). "Brain pathology in pedophilic offenders: Evidence of volume reduction in the right amygdala and related diencephalic structures". Archives of General Psychiatry. 64 (6): 737–746. doi:10.1001/archpsyc.64.6.737. PMID 17548755.
External links[]
- Cerebrum