Tenofovir alafenamide
This article needs to be updated. (January 2021) |
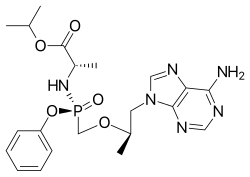 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌtəˈnoʊfəvɪər ˌæləˈfɛnəmaɪd/ |
| Trade names | Vemlidy Genvoya (with elvitegravir, cobicistat and emtricitabine) Odefsey (with emtricitabine and rilpivirine) Descovy (with emtricitabine) Symtuza (with darunavir, cobicistat, and emtricitabine) |
| Other names | GS-7340 |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | ~80%[3] |
| Elimination half-life | 0.51 hour |
| Excretion | Feces (31.7%), urine (<1%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C21H29N6O5P |
| Molar mass | 476.474 g·mol−1 |
| 3D model (JSmol) | |
| |
| |

Tenofovir alafenamide, sold under the brand name Vemlidy, is a hepatitis B virus (HBV) nucleotide reverse transcriptase inhibitor medication for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease.[3] It is taken by mouth.[3]
Tenofovir alafenamide is a prodrug of tenofovir. It was developed by Gilead Sciences based on the protide technology of Chris McGuigan for use in the treatment of HIV/AIDS and chronic hepatitis B, and is applied in the form of tenofovir alafenamide fumarate (TAF). Closely related to the commonly used reverse-transcriptase inhibitor tenofovir disoproxil fumarate (TDF), TAF has greater antiviral activity and better distribution into lymphoid tissues than that agent.[5][6] Vemlidy was approved by the U.S. Food and Drug Administration (FDA) in November 2016.[7]
Gilead announced a Phase III clinical trial evaluating a single-tablet regimen combining tenofovir alafenamide with cobicistat, emtricitabine and elvitegravir[8] and developed a coformulation of the drug with cobicistat, emtricitabine and the protease inhibitor darunavir.[9][10][11] In a 48-week study comparing elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil (trade name Stribild) to elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (trade name Genvoya), the results showed the newer drug's effects to be non-inferior to the established agent, but at much lower dosages and with lower incidence of adverse side effects such as impaired kidney function.[12][13][14] The FDA approved the TAF-based treatment regimen for treatment of HIV-1 in November 2015.[15] Genvoya is the first TAF-based regimen to receive approval.[15]
Fixed-dose combinations containing tenofovir alafenamide[]
- Elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide (brand name Genvoya)[16] — approved both in the United States and in the European Union in November 2015[17][18] (compare Elvitegravir/cobicistat/emtricitabine/tenofovir; brand name Stribild[19][20][21])
- Emtricitabine/rilpivirine/tenofovir alafenamide (brand name Odefsey)[22] — approved in the United States in March 2016, and in the European Union in June 2016[23][24] (compare Emtricitabine/rilpivirine/tenofovir; brand name Complera[25][26])
- Emtricitabine/tenofovir alafenamide (brand name Descovy)[27] — approved in the United States in April 2016 (compare Emtricitabine/tenofovir; brand name Truvada). In October 2019, Descovy was approved in the United States for HIV-1 pre-exposure prophylaxis (PrEP).[28][29]
- Bictegravir/emtricitabine/tenofovir alafenamide (brand name Biktarvy)[30] — approved in the United States in February 2018.
- Darunavir/cobicistat/emtricitabine/tenofovir alafenamide (brand name Symtuza)[31] — approved in the European Union in September 2017, in the United States in July 2018, and in Australia in November 2019.[32][33][34]
- Dolutegravir/emtricitabine/tenofovir alafenamide.[35]
References[]
- ^ a b "Tenofovir alafenamide (Vemlidy) Use During Pregnancy". Drugs.com. 26 December 2018. Retrieved 18 April 2020.
- ^ "Vemlidy 25 mg film coated tablets - Summary of Product Characteristics (SmPC)". (emc). 8 September 2020. Retrieved 12 November 2020.
- ^ a b c d "Vemlidy- tenofovir alafenamide tablet". DailyMed. 11 February 2020. Retrieved 18 April 2020.
- ^ "Vemlidy EPAR". European Medicines Agency (EMA).
- ^ Eisenberg EJ, He GX, Lee WA (2001). "Metabolism of GS-7340, a novel phenyl monophosphoramidate intracellular prodrug of PMPA, in blood". Nucleosides Nucleotides Nucleic Acids. 20 (4–7): 1091–8. doi:10.1081/NCN-100002496. PMID 11562963. S2CID 24652157.
- ^ M Markowitz, A Zolopa, et al. GS-7340 Demonstrates Greater Declines in HIV-1 RNA than Tenofovir Disoproxil Fumarate During 14 Days of Monotherapy in HIV-1 Infected Subjects. 18th Conference on Retroviruses and Opportunistic Infections 2 Mar 2011. Paper # 152LB
- ^ "FDA Approves Vemlidy (tenofovir alafenamide) for Chronic Hepatitis B in Adults". United States Department of Health and Human Services. 21 November 2016. Archived from the original on 11 October 2019. Retrieved 11 October 2019.
- ^ "Gilead Initiates Phase 3 Clinical Program for Tenofovir Alafenamide, a Novel Low-Dose Prodrug for the Treatment of HIV" (Press release). Gilead. 24 January 2013. Archived from the original on 11 October 2019.
- ^ "Gilead Sciences Finalizes Agreement with Tibotec Pharmaceuticals to Develop and Commercialize a Single-Tablet Regimen of Prezista with Emtriva, GS 7340 and Cobicistat". Gilead Sciences (Press release). 15 November 2011. Archived from the original on 11 October 2019. Retrieved 10 October 2019.
- ^ GS-7340 Packs Greater HIV Punch, Potentially Better Safety, Versus Viread Horn, Tim. 15 Mar 2012. AIDSmeds.com
- ^ Pharmacokinetics of a Novel EVG/COBI/FTC/GS-7340 Single Tablet Regimen. 13th International Workshop on Clinical Pharmacology of HIV Therapy. Barcelona, Spain. April 16–18, 2012.
- ^ Once-Daily Tenofovir Prodrug Combo Pill as Effective as Stribild. AIDSmeds.com 1 Nov 2012.
- ^ CROI 2013: New Pro-drug Tenofovir Alafenamide Appears Equally Effective but Better Tolerated. Highleyman, Liz. HIVandHepatitis.com. 6 March 2013.
- ^ Horn, T. et al. Tenefovir Alafenamide Fumarate (TAF) Sign-On Letter to Gilead. 13 June 2013. Treatment Action Group.
- ^ a b "U.S. Food and Drug Administration Approves Gilead's Single Tablet Regimen Genvoya (Elvitegravir, Cobicistat, Emtricitabine and Tenofovir Alafenamide) for Treatment of HIV-1 Infection" (Press release). Gilead. 5 November 2015. Archived from the original on 8 November 2015.
- ^ "Genvoya- elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide tablet". DailyMed. 11 February 2019. Retrieved 18 April 2020.
- ^ "Genvoya (elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide) fixed-dose combination tablet". U.S. Food and Drug Administration (FDA). 8 December 2015. Retrieved 28 July 2020. Lay summary (PDF).
{{cite web}}: Cite uses deprecated parameter|lay-url=(help) - ^ "Genvoya EPAR". European Medicines Agency (EMA). Retrieved 28 July 2020.
- ^ "Drug Approval Package: Stribild (elvitegravir, cobicistat, emtricitabine, tenofovir disoproxil fumarate) Fixed Dose". U.S. Food and Drug Administration (FDA). 10 October 2012. Retrieved 28 July 2020. Lay summary (PDF).
{{cite web}}: Cite uses deprecated parameter|lay-url=(help) - ^ "Stribild- elvitegravir, cobicistat, emtricitabine, and tenofovir disoproxil fumarate tablet, film coated". DailyMed. 28 January 2019. Retrieved 28 July 2020.
- ^ "Stribild EPAR". European Medicines Agency (EMA). Retrieved 28 July 2020.
- ^ "Odefsey- emtricitabine, rilpivirine hydrochloride, and tenofovir alafenamide tablet". DailyMed. 6 December 2019. Retrieved 18 April 2020.
- ^ "Odefsey (emtricitabine, rilpivirine, and tenofovir alafenamide) Tablets". U.S. Food and Drug Administration (FDA). 29 November 2016. Retrieved 28 July 2020.
- ^ "Odefsey EPAR". European Medicines Agency (EMA). Retrieved 28 July 2020.
- ^ "Drug Approval Package: (emtricitabine/rilpivirine/tenofovir disoproxil fumarate) NDA #202123". U.S. Food and Drug Administration (FDA). 6 September 2012. Retrieved 28 July 2020. Lay summary (PDF).
{{cite web}}: Cite uses deprecated parameter|lay-url=(help) - ^ "Complera- emtricitabine, rilpivirine hydrochloride, and tenofovir disoproxil fumarate tablet, film coated". DailyMed. 9 December 2019. Retrieved 28 July 2020.
- ^ "Descovy- emtricitabine and tenofovir alafenamide tablet". DailyMed. 13 January 2020. Retrieved 18 April 2020.
- ^ "FDA approves second drug to prevent HIV infection as part of ongoing efforts to end the HIV epidemic". U.S. Food and Drug Administration (FDA). 3 October 2019. Archived from the original on 11 October 2019. Retrieved 11 October 2019.
- ^ Mandavilli, Apoorva (4 October 2019). "F.D.A. Approves New H.I.V.-Prevention Drug, but Not for Everyone". The New York Times. Retrieved 11 October 2019.
- ^ "Biktarvy- bictegravir sodium, emtricitabine, and tenofovir alafenamide fumarate tablet". DailyMed. 8 August 2019. Retrieved 18 April 2020.
- ^ "Symtuza- darunavir, cobicistat, emtricitabine, and tenofovir alafenamide tablet, film coated". DailyMed. 6 March 2020. Retrieved 18 April 2020.
- ^ "Drug Approval Package: Symtuza (darunavir, cobicistat, emtricitabine, and tenofovir alafenamide)". U.S. Food and Drug Administration (FDA). 11 December 2018. Retrieved 19 August 2020.
- ^ "Symtuza EPAR". European Medicines Agency. Retrieved 19 August 2020.
- ^ http://www.ebs.tga.gov.au/servlet/xmlmillr6?dbid=ebs/PublicHTML/pdfStore.nsf&docid=CCE11C6BC5177A30CA2585AE00423857&agid=(PrintDetailsPublic)&actionid=1
- ^ "Drugs@FDA: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Retrieved 5 December 2020.
External links[]
- "Tenofovir alafenamide". Drug Information Portal. U.S. National Library of Medicine.
- "Tenofovir alafenamide fumarate". Drug Information Portal. U.S. National Library of Medicine.
- Carboxylate esters
- Fixed dose combination (antiretroviral)
- Gilead Sciences
- Isopropyl esters
- Nucleoside analog reverse transcriptase inhibitors
- Organophosphates
- Phenol esters
- Pre-exposure prophylaxis