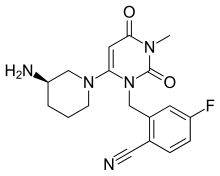
Trelagliptin
 | |
| Clinical data | |
|---|---|
| Trade names | Zafatek, Wedica |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C18H20FN5O2 |
| Molar mass | 357.389 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trelagliptin (trade name Zafatek) is a pharmaceutical drug used for the treatment of type 2 diabetes (diabetes mellitus).[1]
Indications[]
It is a highly selective dipeptidyl peptidase-4 inhibitor that is typically used as an add on treatment when the first line treatment of metformin is not achieving the expected glycemic goals; though it has been approved for use as a first line treatment when metformin cannot be used.[1]
Biochemistry[]
DPP-4 inhibitors activate T-cells and are more commonly known as T-cell activation antigens (specifically CD26).[1][2] Chemically, it is a fluorinated derivative of alogliptin.
Development[]
Formulated as the salt trelagliptin succinate, it was approved for use in Japan in March 2015.[3] Takeda, the company that developed trelagliptin, chose to not get approval for the drug in the US and EU.[1] The licensing rights that Takeda purchased from Furiex Pharmaceuticals for DPP-4 inhibitors included a clause specific to development of this drug in the US and EU.[1] The clause required that all services done for phase II and phase III clinical studies in the US and EU be purchased through Furiex.[1] Takeda chose to cease development of this drug in the US and EU because of the high costs quoted by Furiex for these services.[1] Gliptins have been on the market since 2006 and there are 8 gliptins currently registered as drugs (worldwide).[4] Gliptins are an emerging market and are thus being developed at an increasing rate; there are currently two gliptins in advanced stages of development that are expected to be on the market in the coming year.[4]
Gliptins are thought to have cardiovascular protective abilities though the extent of these effects is still being studied.[4] They are also being studied for the ability that this class of drugs has at promoting B-cell survival.[4]
Administration and dosing[]
Similar drugs in the same class as trelagliptin are administered once daily while trelagliptin is administered once weekly.[1][5] Alogliptin (Nesina) is the other major DPP-4 inhibitor on the market. It is also owned by Takeda and is administered once daily. A dosing of once per week is advantageous as a reduction in the frequency of required dosing is known to increase patient compliance.[1][2]
Brand names[]
In Bangladesh it is marketed under the trade name Wedica.
References[]
- ^ a b c d e f g h i McKeage K (July 2015). "Trelagliptin: First Global Approval". Drugs. 75 (10): 1161–4. doi:10.1007/s40265-015-0431-9. PMID 26115728. S2CID 22120371.
- ^ a b Inagaki N, Onouchi H, Sano H, Funao N, Kuroda S, Kaku K (February 2014). "SYR-472, a novel once-weekly dipeptidyl peptidase-4 (DPP-4) inhibitor, in type 2 diabetes mellitus: a phase 2, randomised, double-blind, placebo-controlled trial". The Lancet. Diabetes & Endocrinology. 2 (2): 125–32. doi:10.1016/s2213-8587(13)70149-9. PMID 24622716.
- ^ "New Drug Application Approval of Zafatek® Tablets for the treatment of Type 2 Diabetes in Japan | Takeda Pharmaceutical Company Limited". www.takeda.com. Retrieved 2015-11-13.
- ^ a b c d Cahn A, Raz I (June 2013). "Emerging gliptins for type 2 diabetes". Expert Opinion on Emerging Drugs. 18 (2): 245–58. doi:10.1517/14728214.2013.807796. PMID 23725569. S2CID 23977578.
- ^ Inagaki N, Onouchi H, Maezawa H, Kuroda S, Kaku K (March 2015). "Once-weekly trelagliptin versus daily alogliptin in Japanese patients with type 2 diabetes: a randomised, double-blind, phase 3, non-inferiority study". The Lancet. Diabetes & Endocrinology. 3 (3): 191–7. doi:10.1016/s2213-8587(14)70251-7. PMID 25609193.
- Drugs not assigned an ATC code
- Dipeptidyl peptidase-4 inhibitors
- Pyrimidinediones
- Fluoroarenes
- Piperidines
- Nitriles