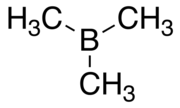
Trimethylborane
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trimethylborane[1] | |||
| Other names
Trimethylborine
Trimethylboron | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| ECHA InfoCard | 100.008.926 | ||
| EC Number |
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| Properties | |||
| C3H9B | |||
| Molar mass | 55.92 g/mol | ||
| Appearance | Colorless gas or liquid | ||
| Density | 0.625 g/cm3 at −100 °C[3] | ||
| Melting point | −161.5 °C (−258.7 °F; 111.6 K) | ||
| Boiling point | −20.2 °C (−4.4 °F; 253.0 K) | ||
| Slight, highly reactive | |||
| Structure | |||
| Δ | |||
| Hazards | |||
| Main hazards | Spontaneously flammable in air; causes burns | ||
| GHS pictograms |   
| ||
| GHS Signal word | Danger | ||
GHS hazard statements
|
H220, H250, H280, H314 | ||
| P210, P222, P260, P264, P280, P301+330+331, P302+334, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P370+378, P377, P381, P403, P405, P410+403, P422, P501 | |||
| Flash point | Not applicable, pyrophoric gas | ||
| −40 °C (−40 °F; 233 K)[4] | |||
| Related compounds | |||
Related compounds
|
| ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trimethylborane (TMB) is a toxic, pyrophoric gas with the formula B(CH3)3 (which can also be written as Me3B, with Me representing methyl).
Properties[]
As a liquid it is colourless. The strongest line in the infrared spectrum is at 1330 cm−1 followed by lines at 3010 cm−1 and 1185 cm−1.
Its melting point is −161.5 °C, and its boiling point is −20.2 °C.
Vapour pressure is given by log P = 6.1385 + 1.75 log T − 1393.3/T − 0.007735 T, where T is temperature in kelvins.[5] Molecular weight is 55.914. The heat of vapourisation is 25.6 kJ/mol.[4]
Preparation[]
Trimethylborane was first described in 1862 by Edward Frankland,[6] who also mentioned its adduct with ammonia.[7] Due to its dangerous nature the compound was no longer studied until 1921, when Alfred Stock and Friedrich Zeidler took advantage of the reaction between boron trichloride gas and dimethylzinc.[8] Although the substance can be prepared using Grignard reagents the output is contaminated by unwanted products from the solvent. Trimethylborane can be made on a small scale with a 98% yield by reacting trimethylaluminium in hexane with boron tribromide in dibutyl ether as a solvent.[5] Yet other methods are reacting with trimethylaluminium chloride, or potassium tetrafluoroborate with trimethylaluminium.[9] Yet another method is to add boron trifluoride in ether to .[10]
Reactions[]
Trimethylborane spontaneously ignites in air if the concentration is high enough. It burns with a green flame producing soot.[11] Slower oxidation with oxygen in a solvent or in the gas phase can produce dimethyltrioxadiboralane, which contains a ring of two boron and three oxygen atoms. However the major product is dimethylborylmethylperoxide, which rapidly decomposes to dimethoxymethylborane.[12]
Trimethylborane is a strong Lewis acid. B(CH3)3 can form an adduct with ammonia: (NH3):B(CH3)3.[13] as well as other Lewis bases. The Lewis acid properties of B(CH3)3 have been analyzed by the ECW model yielding EA= 2.90 and CA= 3.60. When trimethylborane forms an adduct with trimethylamine, steric repulsion between the methyl groups on the B and N results. The ECW model can provide a measure of this steric effect.
Trimethylborane reacts with water and chlorine at room temperature. It also reacts with grease but not with Teflon or glass.[5]
Trimethylborane reacts with diborane to disproportionate to form monomethyldiborane and dimethyldiborane: (CH3)BH2.BH3 and (CH3)2BH.BH3.
It reacts as a gas with trimethylphosphine to form a solid Lewis salt with a heat of formation of −41 kcal per mol. This adduct has a heat of sublimation of −24.6 kcal/mol. No reaction occurs with trimethylarsine or trimethylstibine.[10]
Methyl lithium reacting with the Trimethylborane produces a tetramethylborate salt: LiB(CH3)4.[14] The tetramethylborate ion has a negative charge and is isoelectronic with neopentane, tetramethylsilane, and the tetramethylammonium cation.
Use[]
Trimethylborane has been used as a neutron counter. For this use it has to be very pure.[13] It is also used in chemical vapour deposition where boron and carbon need to be deposited together.
References[]
- ^ "CHAPTER P-6. Applications to Specific Classes of Compounds". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 974. doi:10.1039/9781849733069-00648. ISBN 978-0-85404-182-4.
- ^ Graner, G.; Hirota, E.; Iijima, T.; Kuchitsu, K.; Ramsay, D. A.; Vogt, J.; Vogt, N. (2001). "C3H9B Trimethylborane". Molecules containing Three or Four Carbon Atoms. Landolt-Börnstein - Group II Molecules and Radicals. 25C. p. 1. doi:10.1007/10688787_381. ISBN 978-3-540-66774-2.
- ^ http://www.voltaix.com/images/doc/Msb000_TMB.pdf MSDS from
- ^ Jump up to: a b Trimethylborane
- ^ Jump up to: a b c William S. Rees, Jr. and al (1990). Alvin P. Ginsberg (ed.). Trimethylborane. Inorganic Syntheses. 27. p. 339.
- ^ E. Frankland (1862). "Ueber eine neue Reihe organischer Verbindungen, welche Bor enthalten". Justus Liebigs Ann. Chem. 124: 129–157. doi:10.1002/jlac.18621240102.
- ^ R. Nishiyabu, Y. Kubo, T.D. James and J. S. Fossey (2011). "Boronic acid building blocks: tools for self assembly". Chem. Commun. 47 (4): 1124–1150. doi:10.1039/C0CC02921A. PMID 21113558.CS1 maint: multiple names: authors list (link)
- ^ A. Stock and F. Zeidler (1921). "Zur Kenntnis des Bormethyls und Boräthyls". Ber. Dtsch. Chem. Ges. A/B. 54 (3): 531–541. doi:10.1002/cber.19210540321.
- ^ Roland Köster, Paul Binger, Wilhelm V. Dahlhoff (1973). "A Convenient Preparation of Trimethylborane and Triethylborane". Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 3 (4): 359–367. doi:10.1080/00945717308057281.CS1 maint: multiple names: authors list (link)
- ^ Jump up to: a b Donald Charles Mente (May 1975). "The Reactions of Trimethyl group Va Lewis Bases with simple Boron Lewis Acids" (PDF).
- ^ Herbert Ellern (1968). Military and Civilian Pyrotechnics. Chemical Publishing Company. p. 24. CiteSeerX 10.1.1.137.1104. ISBN 9780820603643.
- ^ Barton, Lawrence; Crump, John M.; Wheatley, Jeffrey B. (June 1974). "Trioxadiborolanes from the oxidation of methyldiborane". Journal of Organometallic Chemistry. 72 (1): C1–C3. doi:10.1016/s0022-328x(00)82027-6.
- ^ Jump up to: a b Gaylon S. Ross; et al. (2 October 1961). "Preparation of High Purity Trimethylborane" (PDF). Journal of Research of the National Bureau of Standards Section A. 66 (1).
- ^ Georg Wittig in 1958
- Alkylboranes
- Gases