Unbiunium
| Unbiunium | ||||||
|---|---|---|---|---|---|---|
| Pronunciation | /ˌuːnbaɪˈuːniəm/ | |||||
| Alternative names | element 121, eka-actinium | |||||
| Unbiunium in the periodic table | ||||||
| ||||||
| Atomic number (Z) | 121 | |||||
| Group | group n/a | |||||
| Period | period 8 | |||||
| Block | g-block | |||||
| Electron configuration | [Og] 8s2 8p1 (predicted)[1] | |||||
| Electrons per shell | 2, 8, 18, 32, 32, 18, 8, 3 (predicted) | |||||
| Physical properties | ||||||
| unknown | ||||||
| Phase at STP | unknown | |||||
| Atomic properties | ||||||
| Oxidation states | (+1), (+3) (predicted)[1][2] | |||||
| Ionization energies |
| |||||
| Other properties | ||||||
| CAS Number | 54500-70-8 | |||||
| History | ||||||
| Naming | IUPAC systematic element name | |||||
Unbiunium, also known as eka-actinium or simply element 121, is the hypothetical chemical element with symbol Ubu and atomic number 121. Unbiunium and Ubu are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon. In the periodic table of the elements, it is expected to be the first of the superactinides, and the third element in the eighth period. It has attracted attention because of some predictions that it may be in the island of stability, although newer calculations expect the island to occur at a slightly lower atomic number, closer to copernicium and flerovium. It is also likely to be the first of a new g-block of elements.
Unbiunium has not yet been synthesized. It is expected to be one of the last few reachable elements with current technology; the limit could be anywhere between element 120 and 124. It will also likely be far more difficult to synthesize than the elements known so far up to 118, and still more difficult than elements 119 and 120. The teams at RIKEN in Japan and at the JINR in Dubna, Russia have plans to attempt the synthesis of element 121 in the future after they attempt elements 119 and 120.
The position of unbiunium in the periodic table suggests that it would have similar properties to lanthanum and actinium; however, relativistic effects may cause some of its properties to differ from those expected from a straight application of periodic trends. For example, unbiunium is expected to have a s2p valence electron configuration, instead of the s2d of lanthanum and actinium or the s2g expected from the Madelung rule, but this is not predicted to affect its chemistry much. It would on the other hand significantly lower its first ionization energy beyond what would be expected from periodic trends.
Introduction[]
| External video | |
|---|---|
The heaviest[a] atomic nuclei are created in nuclear reactions that combine two other nuclei of unequal size[b] into one; roughly, the more unequal the two nuclei in terms of mass, the greater the possibility that the two react.[9] The material made of the heavier nuclei is made into a target, which is then bombarded by the beam of lighter nuclei. Two nuclei can only fuse into one if they approach each other closely enough; normally, nuclei (all positively charged) repel each other due to electrostatic repulsion. The strong interaction can overcome this repulsion but only within a very short distance from a nucleus; beam nuclei are thus greatly accelerated in order to make such repulsion insignificant compared to the velocity of the beam nucleus.[10] Coming close alone is not enough for two nuclei to fuse: when two nuclei approach each other, they usually remain together for approximately 10−20 seconds and then part ways (not necessarily in the same composition as before the reaction) rather than form a single nucleus.[10][11] If fusion does occur, the temporary merger—termed a compound nucleus—is an excited state. To lose its excitation energy and reach a more stable state, a compound nucleus either fissions or ejects one or several neutrons,[c] which carry away the energy. This occurs in approximately 10−16 seconds after the initial collision.[12][d]
The beam passes through the target and reaches the next chamber, the separator; if a new nucleus is produced, it is carried with this beam.[15] In the separator, the newly produced nucleus is separated from other nuclides (that of the original beam and any other reaction products)[e] and transferred to a surface-barrier detector, which stops the nucleus. The exact location of the upcoming impact on the detector is marked; also marked are its energy and the time of the arrival.[15] The transfer takes about 10−6 seconds; in order to be detected, the nucleus must survive this long.[18] The nucleus is recorded again once its decay is registered, and the location, the energy, and the time of the decay are measured.[15]
Stability of a nucleus is provided by the strong interaction. However, its range is very short; as nuclei become larger, their influence on the outermost nucleons (protons and neutrons) weakens. At the same time, the nucleus is torn apart by electrostatic repulsion between protons, as it has unlimited range.[19] Nuclei of the heaviest elements are thus theoretically predicted[20] and have so far been observed[21] to primarily decay via decay modes that are caused by such repulsion: alpha decay and spontaneous fission;[f] these modes are predominant for nuclei of superheavy elements. Alpha decays are registered by the emitted alpha particles, and the decay products are easy to determine before the actual decay; if such a decay or a series of consecutive decays produces a known nucleus, the original product of a reaction can be determined arithmetically.[g] Spontaneous fission, however, produces various nuclei as products, so the original nuclide cannot be determined from its daughters.[h]
The information available to physicists aiming to synthesize one of the heaviest elements is thus the information collected at the detectors: location, energy, and time of arrival of a particle to the detector, and those of its decay. The physicists analyze this data and seek to conclude that it was indeed caused by a new element and could not have been caused by a different nuclide than the one claimed. Often, provided data is insufficient for a conclusion that a new element was definitely created and there is no other explanation for the observed effects; errors in interpreting data have been made.[i]
History[]

Transactinide elements, such as unbiunium, are produced by nuclear fusion. These fusion reactions can be divided into "hot" and "cold" fusion,[j] depending on the excitation energy of the compound nucleus produced. In hot fusion reactions, very light, high-energy projectiles are accelerated toward very heavy targets (actinides), giving rise to compound nuclei at high excitation energies (~40–50 MeV) that may fission or evaporate several (3 to 5) neutrons.[35] In cold fusion reactions (which use heavier projectiles, typically from the fourth period, and lighter targets, usually lead and bismuth), the fused nuclei produced have a relatively low excitation energy (~10–20 MeV), which decreases the probability that these products will undergo fission reactions. As the fused nuclei cool to the ground state, they require emission of only one or two neutrons. However, hot fusion reactions tend to produce more neutron-rich products because the actinides have the highest neutron-to-proton ratios of any element that can presently be made in macroscopic quantities; it is currently the only method to produce the superheavy elements from flerovium (element 114) onward.[36]
Attempts to synthesize elements 119 and 120 push the limits of current technology, due to the decreasing cross sections of the production reactions and their probably short half-lives,[33] expected to be on the order of microseconds.[1][37] Heavier elements, beginning with element 121, would likely be too short-lived to be detected with current technology, decaying within a microsecond before reaching the detectors.[33] Where this one-microsecond border of half-lives lies is not known, and this may allow the synthesis of some isotopes of elements 121 through 124, with the exact limit depending on the model chosen for predicting nuclide masses.[37] It is also possible that element 120 is the last element reachable with current experimental techniques, and that elements from 121 onward will require new methods.[33]
Because of the current impossibility of synthesizing elements beyond californium (Z = 98) in sufficient quantities to create a target, with einsteinium (Z = 99) targets being currently considered, the practical synthesis of elements beyond oganesson requires heavier projectiles, such as titanium-50, chromium-54, iron-58, or nickel-64.[38][39] This, however, has the drawback of resulting in more symmetrical fusion reactions that are colder and less likely to succeed.[38] For example, the reaction between 243Am and 58Fe is expected to have a cross section on the order of 0.5 fb, several orders of magnitude lower than measured cross sections in successful reactions; such an obstacle would make this and similar reactions infeasible for producing unbiunium.[40]
Past synthesis attempt[]
The synthesis of unbiunium was first attempted in 1977 by bombarding a target of uranium-238 with copper-65 ions at the Gesellschaft für Schwerionenforschung in Darmstadt, Germany:
- 238
92U
+ 65
29Cu
→ 303
121Ubu
* → no atoms
No atoms were identified.[41]
Prospects for future synthesis[]
Currently, the beam intensities at superheavy element facilities result in about 1012 projectiles hitting the target per second; this cannot be increased without burning the target and the detector, and producing larger amounts of the increasingly unstable actinides needed for the target is impractical. The team at the Joint Institute for Nuclear Research (JINR) in Dubna is building a new superheavy element factory (SHE-factory) with improved detectors and the ability to work on a smaller scale, but even so, continuing beyond element 120 and perhaps 121 would be a great challenge.[43] It is possible that the age of fusion–evaporation reactions to produce new superheavy elements is coming to an end due to the increasingly short half-lives to spontaneous fission and the looming proton drip line, so that new techniques such as nuclear transfer reactions (for example, firing uranium nuclei at each other and letting them exchange protons, potentially producing products with around 120 protons) would be required to reach the superactinides.[43]
Because the cross sections of these fusion-evaporation reactions increase with the asymmetry of the reaction, titanium would be a better projectile than chromium for the synthesis of element 121,[44] though this necessitates an einsteinium target. This poses severe challenges due to the significant heating and damage of the target due to the high radioactivity of einsteinium-254, but it would nonetheless probably be the most promising approach. It would require working on a smaller scale due to the lower amount of 254Es that can be produced. This small-scale work could in the near future only be carried out in Dubna's SHE-factory.[45]
The isotopes 299Ubu, 300Ubu, and 301Ubu, that could be produced in the reaction between 254Es and 50Ti via the 3n and 4n channels, are expected to be the only reachable unbiunium isotopes with half-lives long enough for detection. The cross sections would nevertheless push the limits of what can currently be detected. For example, the cross section of the aforementioned reaction between 254Es and 50Ti is predicted to be on the order of 7 fb in the 4n channel,[46] four times lower than the lowest measured cross section for a successful reaction. Should such a reaction be successful, though, the resulting nuclei would decay through isotopes of ununennium that could be produced by cross-bombardments in the 248Cm+51V or 249Bk+50Ti reactions, down through known isotopes of tennessine and moscovium synthesized in the 249Bk+48Ca and 243Am+48Ca reactions.[33] The multiplicity of excited states populated by the alpha decay of odd nuclei may however preclude clear cross-bombardment cases, as was seen in the controversial link between 293Ts and 289Mc.[47][48] Heavier isotopes are expected to be more stable; 320Ubu is predicted to be the most stable unbiunium isotope, but there is no way to synthesize it with current technology as no combination of usable target and projectile could provide enough neutrons.[2]
The teams at RIKEN and at JINR have listed the synthesis of element 121 among their future plans.[45][49][50] These two laboratories are best suited to these experiments as they are the only ones in the world where long beam times are accessible for reactions with such low predicted cross-sections.[51]
Naming[]
Using Mendeleev's nomenclature for unnamed and undiscovered elements, unbiunium should be known as eka-actinium. Using the 1979 IUPAC recommendations, the element should be temporarily called unbiunium (symbol Ubu) until it is discovered, the discovery is confirmed, and a permanent name chosen.[52] Although widely used in the chemical community on all levels, from chemistry classrooms to advanced textbooks, the recommendations are mostly ignored among scientists who work theoretically or experimentally on superheavy elements, who call it "element 121", with the symbol E121, (121), or 121.[1]
Nuclear stability and isotopes[]
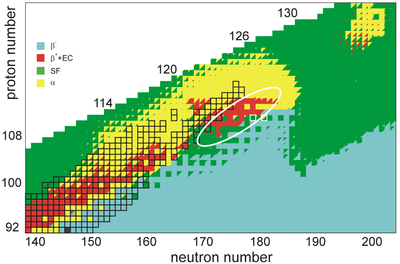
The stability of nuclei decreases greatly with the increase in atomic number after curium, element 96, whose half-life is four orders of magnitude longer than that of any currently known higher-numbered element. All isotopes with an atomic number above 101 undergo radioactive decay with half-lives of less than 30 hours. No elements with atomic numbers above 82 (after lead) have stable isotopes.[53] Nevertheless, for reasons not yet well understood, there is a slight increase of nuclear stability around atomic numbers 110–114, which leads to the appearance of what is known in nuclear physics as the "island of stability". This concept, proposed by University of California professor Glenn Seaborg and stemming from the stabilizing effects of the closed nuclear shells around Z = 114 (or possibly 120, 122, 124, or 126) and N = 184 (and possibly also N = 228), explains why superheavy elements last longer than predicted.[54][55] In fact, the mere existence of elements heavier than rutherfordium can be attested to shell effects and the island of stability, as spontaneous fission would rapidly cause such nuclei to disintegrate in a model neglecting such factors.[56]
A 2016 calculation of the half-lives of the isotopes of unbiunium from 290Ubu to 339Ubu suggested that those from 290Ubu to 303Ubu would not be bound and would decay through proton emission, those from 304Ubu through 314Ubu would undergo alpha decay, and those from 315Ubu to 339Ubu would undergo spontaneous fission. Only the isotopes from 309Ubu to 314Ubu would have long enough alpha-decay lifetimes to be detected in laboratories, starting decay chains terminating in spontaneous fission at moscovium, tennessine, or ununennium. This would present a grave problem for experiments aiming at synthesizing isotopes of unbiunium if true, because the isotopes whose alpha decay could be observed could not be reached by any presently usable combination of target and projectile.[57] Calculations in 2016 and 2017 by the same authors on elements 123 and 125 suggest a less bleak outcome, with alpha decay chains from the more reachable nuclides 300–307Ubt passing through unbiunium and leading down to bohrium or nihonium.[58] It has also been suggested that cluster decay might be a significant decay mode in competition with alpha decay and spontaneous fission in the region past Z = 120, which would pose yet another hurdle for experimental identification of these nuclides.[59][60][61]
Predicted chemistry[]
Unbiunium is predicted to be the first element of an unprecedentedly long transition series, called the superactinides in analogy to the earlier actinides. While its behavior is not likely to be very distinct from lanthanum and actinium,[1] it is likely to pose a limit to the applicability of the periodic law; after element 121, the 5g, 6f, 7d, and 8p1/2 orbitals are expected to fill up together due to their very close energies, and around the elements in the late 150s and 160s, the 9s, 9p1/2, and 8p3/2 subshells join in, so that the chemistry of the elements just beyond 121 and 122 (the last for which complete calculations have been conducted) is expected to be so similar that their position in the periodic table would be purely a formal matter.[62][1]
Based on the Aufbau principle, one would expect the 5g subshell to begin filling at the unbiunium atom. However, while lanthanum does have significant 4f involvement in its chemistry, it does not yet have a 4f electron in its ground-state gas-phase configuration; a greater delay occurs for 5f, where neither actinium nor thorium atoms has a 5f electron although 5f contributes to their chemistry. It is predicted that a similar situation of delayed "radial" collapse might happen for unbiunium so that the 5g orbitals do not start filling until around element 125, even though some 5g chemical involvement may begin earlier. Because of the lack of radial nodes in the 5g orbitals, analogous to the 4f but not the 5f orbitals, the position of unbiunium in the periodic table is expected to be more akin to that of lanthanum than that of actinium among its congeners, and some have proposed to rename the superactinides as "superlanthanides" for that reason.[63] The lack of radial nodes in the 4f orbitals contribute to their core-like behavior in the lanthanide series, unlike the more valence-like 5f orbitals in the actinides; however, the relativistic expansion and destabilization of the 5g orbitals should partially compensate for their lack of radial nodes and hence smaller extent.[64]
Unbiunium is expected to fill the 8p1/2 orbital due to its relativistic stabilization, with a configuration of [Og] 8s2 8p1. Nevertheless, the [Og] 7d1 8s2 configuration, which would be analogous to lanthanum and actinium, is expected to be a low-lying excited state at only 0.412 eV,[65] and the expected [Og] 5g1 8s2 configuration from the Madelung rule should be at 2.48 eV.[66] The electron configurations of the ions of unbiunium are expected to be Ubu+, [Og]8s2; Ubu2+, [Og]8s1; and Ubu3+, [Og].[67] The 8p electron of unbiunium is expected to be very loosely bound, so that its predicted ionization energy of 4.45 eV is lower than that of ununennium (4.53 eV) and all known elements except for the alkali metals from potassium to francium. A similar large reduction in ionization energy is also seen in lawrencium, another element having an anomalous s2p configuration due to relativistic effects.[1]
Despite the change in electron configuration and possibility of using the 5g shell, unbiunium is not expected to behave chemically very differently from lanthanum and actinium. A 2016 calculation on unbiunium monofluoride (UbuF) showed similarities between the valence orbitals of unbiunium in this molecule and those of actinium in actinium monofluoride (AcF); in both molecules, the highest occupied molecular orbital is expected to be non-bonding, unlike in the superficially more similar nihonium monofluoride (NhF) where it is bonding. Nihonium has the electron configuration [Rn] 5f14 6d10 7s2 7p1, with an s2p valence configuration. Unbiunium may hence be somewhat like lawrencium in having an anomalous s2p configuration that does not affect its chemistry: the bond dissociation energies, bond lengths, and polarizabilities of the UbuF molecule are expected to continue the trend through scandium, yttrium, lanthanum, and actinium, all of which have three valence electrons above a noble gas core. The Ubu–F bond is expected to be strong and polarized, just like for the lanthanum and actinium monofluorides.[2]
The non-bonding electrons on unbiunium in UbuF are expected to be able to bond to extra atoms or groups, resulting in the formation of the unbiunium trihalides UbuX3, analogous to LaX3 and AcX3. Hence, the main oxidation state of unbiunium in its compounds should be +3, although the closeness of the valence subshells' energy levels may permit higher oxidation states, just like in elements 119 and 120.[1][2][63] The standard electrode potential for the Ubu3+/Ubu couple is predicted as −2.1 V.[1]
Notes[]
- ^ In nuclear physics, an element is called heavy if its atomic number is high; lead (element 82) is one example of such a heavy element. The term "superheavy elements" typically refers to elements with atomic number greater than 103 (although there are other definitions, such as atomic number greater than 100[4] or 112;[5] sometimes, the term is presented an equivalent to the term "transactinide", which puts an upper limit before the beginning of the hypothetical superactinide series).[6] Terms "heavy isotopes" (of a given element) and "heavy nuclei" mean what could be understood in the common language—isotopes of high mass (for the given element) and nuclei of high mass, respectively.
- ^ In 2009, a team at JINR led by Oganessian published results of their attempt to create hassium in a symmetric 136Xe + 136Xe reaction. They failed to observe a single atom in such a reaction, putting the upper limit on the cross section, the measure of probability of a nuclear reaction, as 2.5 pb.[7] In comparison, the reaction that resulted in hassium discovery, 208Pb + 58Fe, had a cross section of ~20 pb (more specifically, 19+19
−11 pb), as estimated by the discoverers.[8] - ^ The greater the excitation energy, the more neutrons are ejected. If the excitation energy is lower than energy binding each neutron to the rest of the nucleus, neutrons are not emitted; instead, the compound nucleus de-excites by emitting a gamma ray.[12]
- ^ The definition by the IUPAC/IUPAP Joint Working Party states that a chemical element can only be recognized as discovered if a nucleus of it has not decayed within 10−14 seconds. This value was chosen as an estimate of how long it takes a nucleus to acquire its outer electrons and thus display its chemical properties.[13] This figure also marks the generally accepted upper limit for lifetime of a compound nucleus.[14]
- ^ This separation is based on that the resulting nuclei move past the target more slowly then the unreacted beam nuclei. The separator contains electric and magnetic fields whose effects on a moving particle cancel out for a specific velocity of a particle.[16] Such separation can also be aided by a time-of-flight measurement and a recoil energy measurement; a combination of the two may allow to estimate the mass of a nucleus.[17]
- ^ Not all decay modes are caused by electrostatic repulsion. For example, beta decay is caused by the weak interaction.[22]
- ^ Since mass of a nucleus is not measured directly but is rather calculated from that of another nucleus, such measurement is called indirect. Direct measurements are also possible, but for the most part they have remained unavailable for heaviest nuclei.[23] The first direct measurement of mass of a superheavy nucleus was reported in 2018 at LBNL.[24] Mass was determined from the location of a nucleus after the transfer (the location helps determine its trajectory, which is linked to the mass-to-charge ratio of the nucleus, since the transfer was done in presence of a magnet).[25]
- ^ Spontaneous fission was discovered by Soviet physicist Georgy Flerov,[26] a leading scientist at JINR, and thus it was a "hobbyhorse" for the facility.[27] In contrast, the LBL scientists believed fission information was not sufficient for a claim of synthesis of an element. They believed spontaneous fission had not been studied enough to use it for identification of a new element, since there was a difficulty of establishing that a compound nucleus had only ejected neutrons and not charged particles like protons or alpha particles.[14] They thus preferred to link new isotopes to the already known ones by successive alpha decays.[26]
- ^ For instance, element 102 was mistakenly identified in 1957 at the Nobel Institute of Physics in Stockholm, Stockholm County, Sweden.[28] There were no earlier definitive claims of creation of this element, and the element was assigned a name by its Swedish, American, and British discoverers, nobelium. It was later shown that the identification was incorrect.[29] The following year, LBNL was unable to reproduce the Swedish results and announced instead their synthesis of the element; that claim was also disproved later.[29] JINR insisted that they were the first to create the element and suggested a name of their own for the new element, joliotium;[30] the Soviet name was also not accepted (JINR later referred to the naming of element 102 as "hasty").[31] The name "nobelium" remained unchanged on account of its widespread usage.[32]
- ^ Despite the name, "cold fusion" in the context of superheavy element synthesis is a distinct concept from the idea that nuclear fusion can be achieved in room temperature conditions (see cold fusion).[34]
References[]
- ^ a b c d e f g h i j Hoffman, Darleane C.; Lee, Diana M.; Pershina, Valeria (2006). "Transactinides and the future elements". In Morss; Edelstein, Norman M.; Fuger, Jean (eds.). The Chemistry of the Actinide and Transactinide Elements (3rd ed.). Dordrecht, The Netherlands: Springer Science+Business Media. ISBN 978-1-4020-3555-5.
- ^ a b c d Amador, Davi H. T.; de Oliveira, Heibbe C. B.; Sambrano, Julio R.; Gargano, Ricardo; de Macedo, Luiz Guilherme M. (12 September 2016). "4-Component correlated all-electron study on Eka-actinium Fluoride (E121F) including Gaunt interaction: Accurate analytical form, bonding and influence on rovibrational spectra". Chemical Physics Letters. 662: 169–175. Bibcode:2016CPL...662..169A. doi:10.1016/j.cplett.2016.09.025.
- ^ Wakhle, A.; Simenel, C.; Hinde, D. J.; et al. (2015). Simenel, C.; Gomes, P. R. S.; Hinde, D. J.; et al. (eds.). "Comparing Experimental and Theoretical Quasifission Mass Angle Distributions". European Physical Journal Web of Conferences. 86: 00061. Bibcode:2015EPJWC..8600061W. doi:10.1051/epjconf/20158600061. ISSN 2100-014X.
- ^ Krämer, K. (2016). "Explainer: superheavy elements". Chemistry World. Retrieved 2020-03-15.
- ^ "Discovery of Elements 113 and 115". Lawrence Livermore National Laboratory. Archived from the original on 2015-09-11. Retrieved 2020-03-15.
- ^ Eliav, E.; Kaldor, U.; Borschevsky, A. (2018). "Electronic Structure of the Transactinide Atoms". In Scott, R. A. (ed.). Encyclopedia of Inorganic and Bioinorganic Chemistry. John Wiley & Sons. pp. 1–16. doi:10.1002/9781119951438.eibc2632. ISBN 978-1-119-95143-8.
- ^ Oganessian, Yu. Ts.; Dmitriev, S. N.; Yeremin, A. V.; et al. (2009). "Attempt to produce the isotopes of element 108 in the fusion reaction 136Xe + 136Xe". Physical Review C. 79 (2): 024608. doi:10.1103/PhysRevC.79.024608. ISSN 0556-2813.
- ^ Münzenberg, G.; Armbruster, P.; Folger, H.; et al. (1984). "The identification of element 108" (PDF). Zeitschrift für Physik A. 317 (2): 235–236. Bibcode:1984ZPhyA.317..235M. doi:10.1007/BF01421260. Archived from the original (PDF) on 7 June 2015. Retrieved 20 October 2012.
- ^ Subramanian, S. (2019). "Making New Elements Doesn't Pay. Just Ask This Berkeley Scientist". Bloomberg Businessweek. Retrieved 2020-01-18.
- ^ a b Ivanov, D. (2019). "Сверхтяжелые шаги в неизвестное" [Superheavy steps into the unknown]. N+1 (in Russian). Retrieved 2020-02-02.
- ^ Hinde, D. (2014). "Something new and superheavy at the periodic table". The Conversation. Retrieved 2020-01-30.
- ^ a b Krása, A. (2010). "Neutron Sources for ADS" (PDF). Czech Technical University in Prague. pp. 4–8. Archived from the original (PDF) on 2019-03-03. Retrieved October 20, 2019.
- ^ Wapstra, A. H. (1991). "Criteria that must be satisfied for the discovery of a new chemical element to be recognized" (PDF). Pure and Applied Chemistry. 63 (6): 883. doi:10.1351/pac199163060879. ISSN 1365-3075. Retrieved 2020-08-28.
- ^ a b Hyde, E. K.; Hoffman, D. C.; Keller, O. L. (1987). "A History and Analysis of the Discovery of Elements 104 and 105". Radiochimica Acta. 42 (2): 67–68. doi:10.1524/ract.1987.42.2.57. ISSN 2193-3405.
- ^ a b c Chemistry World (2016). "How to Make Superheavy Elements and Finish the Periodic Table [Video]". Scientific American. Retrieved 2020-01-27.
- ^ Hoffman, Ghiorso & Seaborg 2000, p. 334.
- ^ Hoffman, Ghiorso & Seaborg 2000, p. 335.
- ^ Zagrebaev, Karpov & Greiner 2013, p. 3.
- ^ Beiser 2003, p. 432.
- ^ Staszczak, A.; Baran, A.; Nazarewicz, W. (2013). "Spontaneous fission modes and lifetimes of superheavy elements in the nuclear density functional theory". Physical Review C. 87 (2): 024320–1. arXiv:1208.1215. Bibcode:2013PhRvC..87b4320S. doi:10.1103/physrevc.87.024320. ISSN 0556-2813.
- ^ Audi et al. 2017, pp. 030001-128–030001-138.
- ^ Beiser 2003, p. 439.
- ^ Oganessian, Yu. Ts.; Rykaczewski, K. P. (2015). "A beachhead on the island of stability". Physics Today. 68 (8): 32–38. Bibcode:2015PhT....68h..32O. doi:10.1063/PT.3.2880. ISSN 0031-9228. OSTI 1337838.
- ^ Grant, A. (2018). "Weighing the heaviest elements". Physics Today. doi:10.1063/PT.6.1.20181113a.
- ^ Howes, L. (2019). "Exploring the superheavy elements at the end of the periodic table". Chemical & Engineering News. Retrieved 2020-01-27.
- ^ a b Robinson, A. E. (2019). "The Transfermium Wars: Scientific Brawling and Name-Calling during the Cold War". Distillations. Retrieved 2020-02-22.
- ^ "Популярная библиотека химических элементов. Сиборгий (экавольфрам)" [Popular library of chemical elements. Seaborgium (eka-tungsten)]. n-t.ru (in Russian). Retrieved 2020-01-07. Reprinted from "Экавольфрам" [Eka-tungsten]. Популярная библиотека химических элементов. Серебро — Нильсборий и далее [Popular library of chemical elements. Silver through nielsbohrium and beyond] (in Russian). Nauka. 1977.
- ^ "Nobelium – Element information, properties and uses | Periodic Table". Royal Society of Chemistry. Retrieved 2020-03-01.
- ^ a b Kragh 2018, pp. 38–39.
- ^ Kragh 2018, p. 40.
- ^ Ghiorso, A.; Seaborg, G. T.; Oganessian, Yu. Ts.; et al. (1993). "Responses on the report 'Discovery of the Transfermium elements' followed by reply to the responses by Transfermium Working Group" (PDF). Pure and Applied Chemistry. 65 (8): 1815–1824. doi:10.1351/pac199365081815. Archived (PDF) from the original on 25 November 2013. Retrieved 7 September 2016.
- ^ Commission on Nomenclature of Inorganic Chemistry (1997). "Names and symbols of transfermium elements (IUPAC Recommendations 1997)" (PDF). Pure and Applied Chemistry. 69 (12): 2471–2474. doi:10.1351/pac199769122471.
- ^ a b c d e Zagrebaev, Karpov & Greiner 2013.
- ^ Fleischmann, Martin; Pons, Stanley (1989). "Electrochemically induced nuclear fusion of deuterium". Journal of Electroanalytical Chemistry and Interfacial Electrochemistry. 261 (2): 301–308. doi:10.1016/0022-0728(89)80006-3.
- ^ Barber, Robert C.; Gäggeler, Heinz W.; Karol, Paul J.; et al. (2009). "Discovery of the element with atomic number 112 (IUPAC Technical Report)" (PDF). Pure and Applied Chemistry. 81 (7): 1331. doi:10.1351/PAC-REP-08-03-05. S2CID 95703833.
- ^ Armbruster, Peter & Munzenberg, Gottfried (1989). "Creating superheavy elements". Scientific American. 34: 36–42.
- ^ a b c Karpov, Alexander; Zagrebaev, Valery; Greiner, Walter (1 April 2015). "Superheavy Nuclei: which regions of nuclear map are accessible in the nearest studies" (PDF). cyclotron.tamu.edu. Texas A & M University. Retrieved 30 April 2017.
- ^ a b Folden III, C. M.; Mayorov, D. A.; Werke, T. A.; et al. (2013). "Prospects for the discovery of the next new element: Influence of projectiles with Z > 20". Journal of Physics: Conference Series. 420 (1): 012007. arXiv:1209.0498. Bibcode:2013JPhCS.420a2007F. doi:10.1088/1742-6596/420/1/012007. S2CID 119275964.
- ^ Gan, ZaiGuo; Zhou, XiaoHong; Huang, MingHui; et al. (August 2011). "Predictions of synthesizing element 119 and 120". Science China Physics, Mechanics and Astronomy. 54 (1): 61–66. Bibcode:2011SCPMA..54...61G. doi:10.1007/s11433-011-4436-4. S2CID 120154116.
- ^ Jiang, J.; Chai, Q.; Wang, B.; et al. (2013). "Investigation of production cross sections for superheavy nuclei with Z = 116~121 in dinuclear system concept". Nuclear Physics Review. 30 (4): 391–397. doi:10.11804/NuclPhysRev.30.04.391.
- ^ Hofmann, Sigurd (2002). On Beyond Uranium. Taylor & Francis. p. 105. ISBN 978-0-415-28496-7.
- ^ Greiner, Walter (2013). "Nuclei: superheavy–superneutronic–strange–and of antimatter" (PDF). Journal of Physics: Conference Series. 413: 012002. Bibcode:2013JPhCS.413a2002G. doi:10.1088/1742-6596/413/1/012002. Retrieved 30 April 2017.
- ^ a b Krämer, Katrina (29 January 2016). "Beyond element 118: the next row of the periodic table". Chemistry World. Retrieved 30 April 2017.
- ^ Siwek-Wilczyńska, K.; Cap, T.; Wilczyński, J. (April 2010). "How can one synthesize the element Z = 120?". International Journal of Modern Physics E. 19 (4): 500. Bibcode:2010IJMPE..19..500S. doi:10.1142/S021830131001490X.
- ^ a b Roberto, J. B. (31 March 2015). "Actinide Targets for Super-Heavy Element Research" (PDF). cyclotron.tamu.edu. Texas A & M University. Retrieved 28 April 2017.
- ^ Ghahramany, Nader; Ansari, Ahmad (September 2016). "Synthesis and decay process of superheavy nuclei with Z=119-122 via hot fusion reactions" (PDF). European Physical Journal A. 52 (287). doi:10.1140/epja/i2016-16287-6. S2CID 125102374.
- ^ Forsberg, U.; Rudolph, D.; Fahlander, C.; et al. (9 July 2016). "A new assessment of the alleged link between element 115 and element 117 decay chains" (PDF). Physics Letters B. 760 (2016): 293–296. Bibcode:2016PhLB..760..293F. doi:10.1016/j.physletb.2016.07.008. Retrieved 2 April 2016.
- ^ Forsberg, Ulrika; Fahlander, Claes; Rudolph, Dirk (2016). Congruence of decay chains of elements 113, 115, and 117 (PDF). Nobel Symposium NS160 – Chemistry and Physics of Heavy and Superheavy Elements. doi:10.1051/epjconf/201613102003.
- ^ Morita, Kōsuke (5 February 2016). "The Discovery of Element 113". YouTube. Retrieved 28 April 2017.
- ^ Sokolova, Svetlana; Popeko, Andrei (24 May 2021). "How are new chemical elements born?". jinr.ru. JINR. Retrieved 4 November 2021.
JINR is currently building the first factory of superheavy elements in the world to synthesize elements 119, 120 and 121, and to study in depth the properties of previously obtained elements.
- ^ Hagino, Kouichi; Hofmann, Sigurd; Miyatake, Hiroari; Nakahara, Hiromichi (July 2012). "平成23年度 研究業績レビュー(中間レビュー)の実施について" [Implementation of the 2011 Research Achievement Review (Interim Review)] (PDF). www.riken.jp (in Japanese). RIKEN. Archived from the original (PDF) on 2019-03-30. Retrieved 5 May 2017.
- ^ Chatt, J. (1979). "Recommendations for the naming of elements of atomic numbers greater than 100". Pure and Applied Chemistry. 51 (2): 381–384. doi:10.1351/pac197951020381.
- ^ de Marcillac, Pierre; Coron, Noël; Dambier, Gérard; et al. (2003). "Experimental detection of α-particles from the radioactive decay of natural bismuth". Nature. 422 (6934): 876–878. Bibcode:2003Natur.422..876D. doi:10.1038/nature01541. PMID 12712201. S2CID 4415582.
- ^ Considine, Glenn D.; Kulik, Peter H. (2002). Van Nostrand's scientific encyclopedia (9th ed.). Wiley-Interscience. ISBN 978-0-471-33230-5. OCLC 223349096.
- ^ Koura, H.; Chiba, S. (2013). "Single-Particle Levels of Spherical Nuclei in the Superheavy and Extremely Superheavy Mass Region". Journal of the Physical Society of Japan. 82. 014201. Bibcode:2013JPSJ...82a4201K. doi:10.7566/JPSJ.82.014201.
- ^ Möller, P. (2016). "The limits of the nuclear chart set by fission and alpha decay" (PDF). EPJ Web of Conferences. 131: 03002:1–8. Bibcode:2016EPJWC.13103002M. doi:10.1051/epjconf/201613103002.
- ^ Santhosh, K. P.; Nithya, C. (27 September 2016). "Predictions on the alpha decay chains of superheavy nuclei with Z = 121 within the range 290 ≤ A ≤ 339". International Journal of Modern Physics E. 25 (10). 1650079. arXiv:1609.05495. Bibcode:2016IJMPE..2550079S. doi:10.1142/S0218301316500798. S2CID 118657750.
- ^ Santhosh, K. P.; Nithya, C. (28 December 2016). "Theoretical predictions on the decay properties of superheavy nuclei Z = 123 in the region 297 ≤ A ≤ 307". The European Physical Journal A. 52 (371). Bibcode:2016EPJA...52..371S. doi:10.1140/epja/i2016-16371-y. S2CID 125959030.
- ^ Santhosh, K. P.; Sukumaran, Indu (25 January 2017). "Decay of heavy particles from Z = 125 superheavy nuclei in the region A = 295–325 using different versions of proximity potential". International Journal of Modern Physics E. 26 (3). 1750003. Bibcode:2017IJMPE..2650003S. doi:10.1142/S0218301317500033.
- ^ Poenaru, Dorin N.; Gherghescu, R. A.; Greiner, W.; Shakib, Nafiseh (September 2014). How Rare Is Cluster Decay of Superheavy Nuclei?. Nuclear Physics: Present and Future FIAS Interdisciplinary Science Series 2015. doi:10.1007/978-3-319-10199-6_13.
- ^ Poenaru, Dorin N.; Gherghescu, R. A.; Greiner, W. (March 2012). "Cluster decay of superheavy nuclei". Physical Review C. 85 (3): 034615. Bibcode:2012PhRvC..85c4615P. doi:10.1103/PhysRevC.85.034615. Retrieved 2 May 2017.
- ^ Loveland, Walter (2015). "The Quest for Superheavy Elements" (PDF). www.int.washington.edu. 2015 National Nuclear Physics Summer School. Retrieved 1 May 2017.
- ^ a b Pyykkö, Pekka (2011). "A suggested periodic table up to Z ≤ 172, based on Dirac–Fock calculations on atoms and ions". Physical Chemistry Chemical Physics. 13 (1): 161–8. Bibcode:2011PCCP...13..161P. doi:10.1039/c0cp01575j. PMID 20967377.
- ^ Kaupp, Martin (1 December 2006). "The role of radial nodes of atomic orbitals for chemical bonding and the periodic table" (PDF). Journal of Computational Chemistry. 28 (1): 320–5. doi:10.1002/jcc.20522. PMID 17143872. S2CID 12677737. Retrieved 14 October 2016.
- ^ Eliav, Ephraim; Shmulyian, Sergei; Kaldor, Uzi; Ishikawa, Yasuyuki (1998). "Transition energies of lanthanum, actinium, and eka-actinium (element 121)". The Journal of Chemical Physics. 109 (10): 3954. Bibcode:1998JChPh.109.3954E. doi:10.1063/1.476995.
- ^ Umemoto, Koichiro; Saito, Susumu (1996). "Electronic Configurations of Superheavy Elements". Journal of the Physical Society of Japan. 65 (10): 3175–3179. doi:10.1143/JPSJ.65.3175. Retrieved 31 January 2021.
- ^ Dolg, Michael (2015). Computational Methods in Lanthanide and Actinide Chemistry. John Wiley & Sons. p. 35. ISBN 978-1-118-68829-8.
Bibliography[]
- Audi, G.; Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S. (2017). "The NUBASE2016 evaluation of nuclear properties" (PDF). Chinese Physics C. 41 (3): 030001. Bibcode:2017ChPhC..41c0001A. doi:10.1088/1674-1137/41/3/030001.
- Beiser, A. (2003). Concepts of modern physics (6th ed.). McGraw-Hill. ISBN 978-0-07-244848-1. OCLC 48965418.
- Hoffman, D. C.; Ghiorso, A.; Seaborg, G. T. (2000). The Transuranium People: The Inside Story. World Scientific. ISBN 978-1-78-326244-1.
- Kragh, H. (2018). From Transuranic to Superheavy Elements: A Story of Dispute and Creation. Springer. ISBN 978-3-319-75813-8.
- Zagrebaev, V.; Karpov, A.; Greiner, W. (2013). "Future of superheavy element research: Which nuclei could be synthesized within the next few years?" (PDF). Journal of Physics: Conference Series. 420. 012001. arXiv:1207.5700. Bibcode:2013JPhCS.420a2001Z. doi:10.1088/1742-6596/420/1/012001. ISSN 1742-6588. S2CID 55434734.
Further reading[]
- Kaldor, U. (2005). "Superheavy Elements—Chemistry and Spectroscopy". Encyclopedia of Computational Chemistry. doi:10.1002/0470845015.cu0044. ISBN 978-0-470-84501-1.
- Seaborg, G. T. (1968). "Elements Beyond 100, Present Status and Future Prospects". Annual Review of Nuclear Science. 18: 53–15. Bibcode:1968ARNPS..18...53S. doi:10.1146/annurev.ns.18.120168.000413.
- Hypothetical chemical elements