WAY-204688
 | |
| Clinical data | |
|---|---|
| Drug class | Nonsteroidal estrogen; Nuclear factor κB inhibitor |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
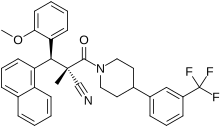
| Formula | C34H31F3N2O2 |
| Molar mass | 556.629 g·mol−1 |
| 3D model (JSmol) | |
| |
InChI
| |
WAY-204688, also known as SIM-688, is a synthetic nonsteroidal estrogen and nuclear factor κB (NF-κB) inhibitor which was originated by ArQule and Wyeth and was under development by Wyeth for the treatment of rheumatoid arthritis, non-specific inflammation, and sepsis but was never marketed.[1][2][3] It is a "pathway-selective" estrogen receptor (ER) ligand which inhibits NF-κB with an IC50 of 122 nM and with maximal inhibition relative to estradiol of 94%.[3][4] Inhibition of NF-κB by WAY-204688 appears to be dependent on agonism of the ERα, as it is reversed by the ERα antagonist fulvestrant, but is not dependent on the ERβ.[3][4] In contrast to the case of NF-κB inhibition, WAY-204688 produces only slight elevation of creatine kinase in vitro, a measure of classical estradiol effects.[3][4] It reached phase I clinical trials prior to the discontinuation of its development.[1]
References[]
- ^ a b "SIM 688 - AdisInsight".
- ^ Ivanenkov YA, Balakin KV, Lavrovsky Y (January 2011). "Small molecule inhibitors of NF-kB and JAK/STAT signal transduction pathways as promising anti-inflammatory therapeutics". Mini Rev Med Chem. 11 (1): 55–78. doi:10.2174/138955711793564079. PMID 21034406.
- ^ a b c d Dodge, Jeffrey A.; Richardson, Timothy I. (2007). "Chapter 10 Novel Selective Estrogen Receptor Modulators (SERMs)". Annual Reports in Medicinal Chemistry Volume 42. Annual Reports in Medicinal Chemistry. Vol. 42. pp. 147–160. doi:10.1016/S0065-7743(07)42010-3. ISBN 9780123739124. ISSN 0065-7743.
- ^ a b c Opal SM, Palardy JE, Cristofaro P, Parejo N, Jhung JW, Keith JC, Chippari S, Caggiano TJ, Steffan RJ, Chadwick CC, Harnish DC (December 2005). "The activity of pathway-selective estrogen receptor ligands in experimental septic shock". Shock. 24 (6): 535–40. doi:10.1097/01.shk.0000183388.90895.cb. PMID 16317384. S2CID 12169698.
External links[]
- 4-Phenylpiperidines
- Abandoned drugs
- Biased ligands
- Ethers
- Ketones
- Naphthalenes
- Nitriles
- Synthetic estrogens
- Trifluoromethyl compounds
- Genito-urinary system drug stubs