Benazepril
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /bəˈnæzəprɪl/ |
| Trade names | Lotensin, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a692011 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 96.7% |
| Metabolism | Liver glucuronidation |
| Elimination half-life | 10-11 hours |
| Excretion | Kidney and biliary |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
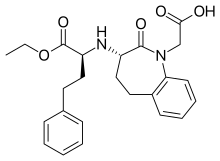
| Formula | C24H28N2O5 |
| Molar mass | 424.497 g·mol−1 |
| 3D model (JSmol) | |
Benazepril, sold under the brand name Lotensin among others, is a medication used to treat high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] It is taken by mouth.[1] Versions are available as the combinations benazepril/hydrochlorothiazide and benazepril/amlodipine.[1]
Common side effects include feeling tired, dizziness, cough, and light-headedness with standing.[1] Serious side effects may include kidney problems, low blood pressure, high blood potassium, and angioedema.[1] Use in pregnancy may harm the baby, while use when breastfeeding may be safe.[2] It is an ACE inhibitor and works by decreasing renin-angiotensin-aldosterone system activity.[1]
Benazepril was patented in 1981 and came into medical use in 1990. It was created by the chemist Mahesh Desai[3] It is available as a generic medication.[1] In 2018, it was the 118th most commonly prescribed medication in the US, with more than 5 million prescriptions.[4][5]
Medical uses[]
It is useful for high blood pressure, heart failure, and diabetic kidney disease.[1] It is a reasonable initial treatment for high blood pressure.[1] Other reasonable initial options include angiotensin II receptor antagonists, calcium-channel blockers, and thiazide diuretics.[1]
Side effects[]
The most common side effects patients experience are a headache or a chronic cough. The chronic cough develops in about 20% of patients treated,[6] and those patients that experience it find it develops after a few months of use. Anaphylaxis, angioedema, and elevation of potassium levels are more serious side effects that can also occur.
Contraindications[]
Benazepril should be discontinued during pregnancy and in women planning to become pregnant, as it can harm the fetus.[7]
Dosage forms[]
It is also available in combination with hydrochlorothiazide, under the trade name Lotensin HCT, and with amlodipine (Lotrel).
Veterinary use[]
Under the brand names Fortekor (Novartis) and VetACE (Jurox Animal Health),[citation needed] benazepril is used to treat congestive heart failure in dogs[8][9] and chronic kidney failure in cats and dogs.[10]
References[]
- ^ Jump up to: a b c d e f g h i j k "Benazepril Hydrochloride Monograph for Professionals". Drugs.com. American Society of Health-System Pharmacists. Retrieved 3 March 2019.
- ^ "Benazepril Pregnancy and Breastfeeding Warnings". Drugs.com. Retrieved 3 March 2019.
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 468. ISBN 9783527607495.
- ^ "The Top 300 of 2021". ClinCalc. Retrieved 18 February 2021.
- ^ "Benazepril Hydrochloride - Drug Usage Statistics". ClinCalc. Retrieved 18 February 2021.
- ^ Dykewicz, Mark S. (April 2004). "Cough and Angioedema From Angiotensin-Converting Enzyme Inhibitors: New Insights Into Mechanisms and Management". Medscape. Retrieved 2 April 2014.
- ^ "Lotensin package insert" (PDF). FDA. 2011. Retrieved 2020-12-09.
- ^ King JN, Mauron C, Kaiser G (December 1995). "Pharmacokinetics of the active metabolite of benazepril, benazeprilat, and inhibition of plasma angiotensin-converting enzyme activity after single and repeated administrations to dogs". Am. J. Vet. Res. 56 (12): 1620–8. PMID 8599524.
- ^ O'Grady MR, O'Sullivan ML, Minors SL, Horne R (2009). "Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers". J. Vet. Intern. Med. 23 (5): 977–83. doi:10.1111/j.1939-1676.2009.0346.x. PMID 19572914.
- ^ "Fortekor Flavor Tabs (5 mg) (Canada) for Animal Use". Drugs.com. Retrieved 2020-12-09.
External links[]
- "Benazepril". Drug Information Portal. U.S. National Library of Medicine.
- ACE inhibitors
- Acetic acids
- Benzazepines
- Enantiopure drugs
- Ethyl esters
- Epsilon-lactams
- Prodrugs
- Carboxylate esters
- Novartis brands