Candesartan
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌkændɪˈsɑːrtən/ |
| Trade names | Atacand, others |
| Other names | Candesartan cilexetil |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a601033 |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 15% (candesartan cilexetil) |
| Metabolism | Candesartan cilexetil: intestinal wall; candesartan: hepatic (CYP2C9) |
| Elimination half-life | 9 hours |
| Excretion | Kidney 33%, faecal 67% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.132.654 |
| Chemical and physical data | |
| Formula | C24H20N6O3 |
| Molar mass | 440.463 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Candesartan is an angiotensin receptor blocker used mainly for the treatment of high blood pressure and congestive heart failure. Candesartan has a very low maintenance dose. The metabolism for the drug is unique as it is a cascading pro-drug. Candesartan has good bioavailibility and is more potent among the AT-1 receptor antagonists.
It was patented in 1990 and approved for medical use in 1997.[1]
Medical uses[]
Hypertension[]
As with other angiotensin II receptor antagonists, candesartan is indicated for the treatment of hypertension.[2] Candesartan has an additive hypertensive effect when combined with a diuretic, such as chlorthalidone. It is available in a fixed-combination formulation with a low dose of the thiazide diuretic hydrochlorothiazide. Candesartan/hydrochlorothiazide combination preparations are marketed under various trade names including Atacand Plus, Hytacand, Blopress Plus, Advantec and Ratacand Plus.
Congestive heart failure[]
Angiotensin receptor blockers such as candesartan and valsartan have been demonstrated in randomised controlled trials to reduce heart failure hospitalisations and cardiovascular deaths for chronic heart failure patients with reduced left ventricular ejection fraction (LVEF ≤40%) and are intolerant to angiotensin-converting enzyme inhibitors.[3][4][5]
Prehypertension[]
In a four-year randomized controlled trial, candesartan was compared to placebo to see whether it could prevent or postpone the development of full-blown hypertension in people with so-called prehypertension. During the first two years of the trial, half of participants were given candesartan while the other half received placebo; candesartan reduced the risk of developing hypertension by nearly two-thirds during this period. In the last two years of the study, all participants were switched to placebo. By the end of the study, candesartan had significantly reduced the risk of hypertension, by more than 15%. Serious adverse effects were more common among participants receiving placebo than in those given candesartan.[6]
Prevention of atrial fibrillation[]
Results from a meta-analysis completed in 2005 demonstrated a reduction in atrial fibrillation in patients with systolic left ventricular dysfunction treated with candesartan, another angiotensin receptor blocker or an angiotensin converting enzyme inhibitor.[7] Evidence for the use of candesartan specifically is also supported by an analysis of the CHARM study which demonstrated a reduction in atrial fibrillation in patients with systolic left ventricular dysfunction.[8] While these studies have demonstrated a potential additional benefit for candesartan when used in patients with systolic left ventricular dysfunction, additional studies are required to further elucidate the role of candesartan in the prevention of atrial fibrillation in other population groups.
Diabetic retinopathy[]
Use of antihypertensive drugs has been demonstrated to slow the progression of diabetic retinopathy; the role of candesartan specifically in reducing progression in type 1 and type 2 diabetes is still up for debate.[9][10][11] Results from a 2008 study on patients with type 1 diabetes showed there was no benefit in using candesartan to reduce progression of diabetic retinopathy when compared to placebo.[10] Candesartan has been demonstrated to reverse the severity (cause regression) of mild to moderate diabetic retinopathy in patients with type 2 diabetes.[11] The patient populations investigated in these studies were limited to mostly Caucasians and those younger than 75 years of age, so generalization of these findings to other population groups should be done with caution.[10][11]
Migraine prophylaxis[]
In two small randomised, controlled cross-over studies, candesartan was shown to be effective for the prophylaxis of migraine,[12][13] however, further studies would enhance confidence in its use for this indication.
Adverse effects[]
As with other drugs that inhibit the renin–angiotensin system, if candesartan is taken by pregnant women during the second or third trimester, it can cause injury and in some cases, death of the developing fetus. Symptomatic hypotension may occur in people who take candesartan and are volume-depleted or salt-depleted, as can also occur when diuretics are coadministered. Reduction in renal glomerular filtration rate may occur; people with renal artery stenosis may be at higher risk. Hyperkalemia may occur; people who are also taking spironolactone or eplerenone may be at higher risk.[2]
Anemia may occur, due to inhibition of the renin–angiotensin system.[14]
As with other angiotensin receptor blockers, candesartan can rarely cause severe liver injury.[15]
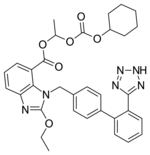
Chemistry and pharmacokinetics[]
Candesartan is marketed as the cyclohexyl 1-hydroxyethyl carbonate (cilexetil) ester, known candesartan cilexetil. Candesartan cilexetil is metabolised completely by esterases in the intestinal wall during absorption to the active candesartan moiety. The first step that occurs in the cascading pro-drug mechanism of Candesartan is that the carbonate gets hydrolyzed. The carbonate gets hydrolyzed and releases carbon dioxide. The metabolite at this step is cyclohexanol, and this is a relatively non-toxic compound which is advantageous to the design of the drug. The other aspect of the cascading prodrug is the O-CH-CH3 molecule which becomes converted into acetic acid, which is another product from the cascading side reaction. Similar to the insight from cyclohexanol, the metabolite of acetic acid relatively is non-toxic and thus less of a hazard if produced as the drug takes pharmacologic action.
The use of a prodrug form increases the bioavailability of candesartan. Despite this, absolute bioavailability is relatively poor at 15% (candesartan cilexetil tablets) to 40% (candesartan cilexetil solution). Its IC50 is 15 μg/kg. Candesartan is not administered in its active form because the administration of the pro-drug would require greater doses and has an unfavorable adverse event profile.
History[]
The compound known as TCV-116 (candesartan) was studied by Japanese scientists using standard laboratory rats. Animal studies were published showing the effectiveness of the compound in 1992–1993, with a pilot study on humans published in the summer of 1993.[16][17]
Names[]
The prodrug candesartan cilexetil is marketed by AstraZeneca and Takeda Pharmaceuticals, commonly under the trade names Blopress, Atacand, Amias, and Ratacand. It is available in generic form.
References[]
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 471. ISBN 9783527607495.
- ^ a b "Candesartan label" (PDF). FDA. February 2016. For label updates see FDA index page for IND 020838
- ^ Pfeffer, Marc A; Swedberg, Karl; Granger, Christopher B; Held, Peter; McMurray, John JV; Michelson, Eric L; Olofsson, Bertil; Östergren, Jan; Yusuf, Salim (September 2003). "Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme". The Lancet. 362 (9386): 759–766. doi:10.1016/s0140-6736(03)14282-1. ISSN 0140-6736.
- ^ Yusuf, Salim; Pfeffer, Marc A; Swedberg, Karl; Granger, Christopher B; Held, Peter; McMurray, John JV; Michelson, Eric L; Olofsson, Bertil; Östergren, Jan (September 2003). "Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial". The Lancet. 362 (9386): 777–781. doi:10.1016/s0140-6736(03)14285-7. ISSN 0140-6736.
- ^ Cohn, Jay N.; Tognoni, Gianni (2001-12-06). "A Randomized Trial of the Angiotensin-Receptor Blocker Valsartan in Chronic Heart Failure". New England Journal of Medicine. 345 (23): 1667–1675. doi:10.1056/NEJMoa010713. ISSN 0028-4793. PMID 11759645.
- ^ Julius S, Nesbitt SD, Egan BM, et al. (July 2006). "Feasibility of treating prehypertension with an angiotensin-receptor blocker". New England Journal of Medicine. 354 (16): 1685–97. doi:10.1056/NEJMoa060838. PMID 16537662.
- ^ Healey, Jeff S., Adrian Baranchuk, Eugene Crystal, Carlos A. Morillo, Michael Garfinkle, Salim Yusuf, and Stuart J. Connolly. "Prevention of atrial fibrillation with angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: a meta-analysis." Journal of the American College of Cardiology 45, no. 11 (2005): 1832-1839.
- ^ Ducharme, Anique; Swedberg, Karl; Pfeffer, Marc A.; Cohen-Solal, Alain; Granger, Christopher B.; Maggioni, Aldo P.; Michelson, Eric L.; McMurray, John J.V.; Olsson, Lars; Rouleau, Jean L.; Young, James B. (July 2006). "Prevention of atrial fibrillation in patients with symptomatic chronic heart failure by candesartan in the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program". American Heart Journal. 152 (1): 86–92. doi:10.1016/j.ahj.2005.06.036.
- ^ Elkjaer AS, Lynge SK, Grauslund J (February 2020). "Evidence and indications for systemic treatment in diabetic retinopathy: a systematic review". Acta Ophthalmol. 98 (4): 329–336. doi:10.1111/aos.14377. PMID 32100477. S2CID 211523341.
- ^ a b c Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. The Lancet. 2008;372(9647):1394-402.
- ^ a b c Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. The Lancet. 2008;372(9647):1385-93.
- ^ Dorosch T, Ganzer CA, Lin M, Seifan A (September 2019). "Efficacy of Angiotensin-Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in the Preventative Treatment of Episodic Migraine in Adults". Curr Pain Headache Rep. 23 (11): 85. doi:10.1007/s11916-019-0823-8. PMID 31515634. S2CID 202557362.
- ^ Cernes R, Mashavi M, Zimlichman R (2011). "Differential clinical profile of candesartan compared to other angiotensin receptor blockers". Vasc Health Risk Manag. 7: 749–59. doi:10.2147/VHRM.S22591. PMC 3253768. PMID 22241949.
- ^ Cheungpasitporn, W; Thongprayoon, C; Chiasakul, T; Korpaisarn, S; Erickson, SB (November 2015). "Renin-angiotensin system inhibitors linked to anemia: a systematic review and meta-analysis". QJM: Monthly Journal of the Association of Physicians. 108 (11): 879–84. doi:10.1093/qjmed/hcv049. PMID 25697787.

- ^ Patti R, Sinha A, Sharma S, Yoon TS, Kupfer Y (May 2019). "Losartan-induced Severe Hepatic Injury: A Case Report and Literature Review". Cureus. 11 (5): e4769. doi:10.7759/cureus.4769. PMC 6663042. PMID 31363450.
- ^ Mizuno, K.; et al. (1992). "Hypotensive activity of TCV-116, a newly developed angiotensin II receptor antagonist, in spontaneously hypertensive rats". Life Sci. 51 (20): PL183-187. doi:10.1016/0024-3205(92)90627-2. PMID 1435062.
- ^ Ogihara, T.; et al. (Jul–Aug 1993). "Pilot study of a new angiotensin II receptor antagonist, TCV-116: effects of a single oral dose on blood pressure in patients with essential hypertension". Clin. Ther. 15 (4): 684–91. PMID 8221818.
- Angiotensin II receptor antagonists
- Tetrazoles
- Benzimidazoles
- Ethers
- Carboxylic acids
- Biphenyls
- AstraZeneca brands
- Takeda Pharmaceutical Company brands