Biohydrogen

Biohydrogen is H2 that is produced biologically.[1] Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass.[2]
Many challenges characterize this technology, including those intrinsic to H2, such as storage and transportation of a noncondensible gas. Hydrogen producing organisms are poisoned by O2. Yields of H2 are often low.
Biochemical principles[]
The main reactions involve fermentation of sugars. Important reactions start with glucose, which is converted to acetic acid:[3]
A related reaction gives formate instead of carbon dioxide:
These reactions are exergonic by 216 and 209 kcal/mol, respectively.
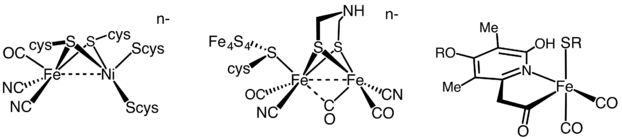
H2 production is catalyzed by two hydrogenases. One is called [FeFe]-hydrogenase; the other is called [NiFe]-hydrogenase. Many organisms express these enzymes. Notable examples are members of the genera Clostridium, Desulfovibrio, Ralstonia, and the pathogen Helicobacter. E. coli is the workhorse for genetic engineering of hydrogenases.[4]
It has been estimated that 99% of all organisms utilize dihydrogen (H2). Most of these species are microbes and their ability to use H2 as a metabolite arises from the expression of H2 metalloenzymes known as hydrogenases.[5] Hydrogenases are sub-classified into three different types based on the active site metal content: iron-iron hydrogenase, nickel-iron hydrogenase, and iron hydrogenase.
Production by algae[]
The biological hydrogen production with algae is a method of photobiological water splitting which is done in a closed photobioreactor based on the production of hydrogen as a solar fuel by algae.[6][7] Algae produce hydrogen under certain conditions. In 2000 it was discovered that if C. reinhardtii algae are deprived of sulfur they will switch from the production of oxygen, as in normal photosynthesis, to the production of hydrogen.[8][9][10]
Photosynthesis[]
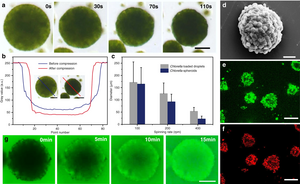
Photosynthesis in cyanobacteria and green algae splits water into hydrogen ions and electrons. The electrons are transported over ferredoxins.[12] Fe-Fe-hydrogenases (enzymes) combine them into hydrogen gas. In Chlamydomonas reinhardtii Photosystem II produces in direct conversion of sunlight 80% of the electrons that end up in the hydrogen gas.[13] Light-harvesting complex photosystem II light-harvesting protein LHCBM9 promotes efficient light energy dissipation.[14] The Fe-Fe-hydrogenases need an anaerobic environment as they are inactivated by oxygen. Fourier transform infrared spectroscopy is used to examine metabolic pathways.[15] In 2020 scientists reported the development of algal-cell based micro-droplets for multicellular spheroid microbial reactors capable of producing hydrogen alongside either oxygen or CO2 via photosynthesis in daylight under air. Enclosing the microreactors with synergistic bacteria was shown to increase levels of hydrogen production.[16][11]
Specialized chlorophyll[]
The chlorophyll (Chl) antenna size in green algae is minimized, or truncated, to maximize photobiological solar conversion efficiency and H2 production. The truncated Chl antenna size minimizes absorption and wasteful dissipation of sunlight by individual cells, resulting in better light utilization efficiency and greater photosynthetic productivity by the green alga mass culture.[17]
Economics[]
It would take about 25,000 square kilometre algal farming to produce biohydrogen equivalent to the energy provided by gasoline in the US alone. This area represents approximately 10% of the area devoted to growing soya in the US.[18]
Bioreactor design issues[]
- Restriction of photosynthetic hydrogen production by accumulation of a proton gradient.
- Competitive inhibition of photosynthetic hydrogen production by carbon dioxide.
- Requirement for bicarbonate binding at photosystem II (PSII) for efficient photosynthetic activity.
- Competitive drainage of electrons by oxygen in algal hydrogen production.
- Economics must reach competitive price to other sources of energy and the economics are dependent on several parameters.
- A major technical obstacle is the efficiency in converting solar energy into chemical energy stored in molecular hydrogen.
Attempts are in progress to solve these problems via bioengineering.
History[]
In 1933, Marjory Stephenson and her student Stickland reported that cell suspensions catalysed the reduction of methylene blue with H2. Six years later, Hans Gaffron observed that the green photosynthetic alga Chlamydomonas reinhardtii, would sometimes produce hydrogen.[19] In the late 1990s Anastasios Melis discovered that deprivation of sulfur induces the alga to switch from the production of oxygen (normal photosynthesis) to the production of hydrogen. He found that the enzyme responsible for this reaction is hydrogenase, but that the hydrogenase lost this function in the presence of oxygen. Melis also discovered that depleting the amount of sulfur available to the algae interrupted their internal oxygen flow, allowing the hydrogenase an environment in which it can react, causing the algae to produce hydrogen.[20] Chlamydomonas moewusii is also a promising strain for the production of hydrogen.[21][22]
Industrial hydrogen[]
Competing for biohydrogen, at least for commercial applications, are many mature industrial processes. Steam reforming of natural gas - sometimes referred to as steam methane reforming (SMR) - is the most common method of producing bulk hydrogen at about 95% of the world production.[23][24][25]
See also[]
References[]
- ^ M. Rögner, ed. (2015). Biohydrogen. De Gruyter. ISBN 978-3-11-033673-3.
- ^ Y.-H. Percival Zhang "Hydrogen Production from Carbohydrates: A Mini-Review" in "Sustainable Production of Fuels, Chemicals, and Fibers from Forest Biomass" ACS Symposium Series, 2011, Volume 1067, pages=203-216.
- ^ Thauer, R. K. (1998). "Biochemistry of Methanogenesis: a Tribute to Marjory Stephenson". Microbiology. 144: 2377–2406. doi:10.1099/00221287-144-9-2377. PMID 9782487.
- ^ Cammack, R.; Frey, M.; Robson, R. (2001). Hydrogen as a Fuel: Learning from Nature. London: Taylor & Francis.
{{cite book}}: CS1 maint: uses authors parameter (link) - ^ Lubitz, Wolfgang; Ogata, Hideaki; Rüdiger, Olaf; Reijerse, Edward (2014). "Hydrogenases". Chemical Reviews. 114 (8): 4081–148. doi:10.1021/cr4005814. PMID 24655035.
- ^ 2013 - Gimpel JA, et al Advances in microalgae engineering and synthetic biology applications for biofuel production
- ^ Hemschemeier, Anja; Melis, Anastasios; Happe, Thomas (2009). "Analytical approaches to photobiological hydrogen production in unicellular green algae". Photosynthesis Research. 102 (2–3): 523–540. doi:10.1007/s11120-009-9415-5. ISSN 0166-8595. PMC 2777220. PMID 19291418.
- ^ Wired-Mutant Algae Is Hydrogen Factory Archived August 27, 2006, at the Wayback Machine
- ^ "Archived copy". Archived from the original on 2008-10-31. Retrieved 2009-03-11.
{{cite web}}: CS1 maint: archived copy as title (link) - ^ Melis, Anastasios; Zhang, Liping; Forestier, Marc; Ghirardi, Maria L.; Seibert, Michael (2000-01-01). "Sustained Photobiological Hydrogen Gas Production upon Reversible Inactivation of Oxygen Evolution in the Green AlgaChlamydomonas reinhardtii". Plant Physiology. 122 (1): 127–136. doi:10.1104/pp.122.1.127. ISSN 1532-2548. PMC 58851. PMID 10631256.
- ^ a b Xu, Zhijun; Wang, Shengliang; Zhao, Chunyu; Li, Shangsong; Liu, Xiaoman; Wang, Lei; Li, Mei; Huang, Xin; Mann, Stephen (25 November 2020). "Photosynthetic hydrogen production by droplet-based microbial micro-reactors under aerobic conditions". Nature Communications. 11 (1): 5985. doi:10.1038/s41467-020-19823-5. ISSN 2041-1723. PMC 7689460. PMID 33239636.
 Available under CC BY 4.0.
Available under CC BY 4.0.
- ^ Peden, E. A.; Boehm, M.; Mulder, D. W.; Davis, R.; Old, W. M.; King, P. W.; Ghirardi, M. L.; Dubini, A. (2013). "Identification of Global Ferredoxin Interaction Networks in Chlamydomonas reinhardtii". Journal of Biological Chemistry. 288 (49): 35192–35209. doi:10.1074/jbc.M113.483727. ISSN 0021-9258. PMC 3853270. PMID 24100040.
- ^ Volgusheva, A.; Styring, S.; Mamedov, F. (2013). "Increased photosystem II stability promotes H2 production in sulfur-deprived Chlamydomonas reinhardtii". Proceedings of the National Academy of Sciences. 110 (18): 7223–7228. Bibcode:2013PNAS..110.7223V. doi:10.1073/pnas.1220645110. ISSN 0027-8424. PMC 3645517. PMID 23589846.
- ^ Grewe, S.; Ballottari, M.; Alcocer, M.; D'Andrea, C.; Blifernez-Klassen, O.; Hankamer, B.; Mussgnug, J. H.; Bassi, R.; Kruse, O. (2014). "Light-Harvesting Complex Protein LHCBM9 Is Critical for Photosystem II Activity and Hydrogen Production in Chlamydomonas reinhardtii". The Plant Cell. 26 (4): 1598–1611. doi:10.1105/tpc.114.124198. ISSN 1040-4651. PMC 4036574. PMID 24706511.
- ^ Langner, U; Jakob, T; Stehfest, K; Wilhelm, C (2009). "An energy balance from absorbed photons to new biomass for Chlamydomonas reinhardtii and Chlamydomonas acidophila under neutral and extremely acidic growth conditions". Plant Cell Environ. 32 (3): 250–8. doi:10.1111/j.1365-3040.2008.01917.x. PMID 19054351.
- ^ "Research creates hydrogen-producing living droplets, paving way for alternative future energy source". phys.org. Retrieved 9 December 2020.
- ^ Kirst, H.; Garcia-Cerdan, J. G.; Zurbriggen, A.; Ruehle, T.; Melis, A. (2012). "Truncated Photosystem Chlorophyll Antenna Size in the Green Microalga Chlamydomonas reinhardtii upon Deletion of the TLA3-CpSRP43 Gene". Plant Physiology. 160 (4): 2251–2260. doi:10.1104/pp.112.206672. ISSN 0032-0889. PMC 3510145. PMID 23043081.
- ^ Growing hydrogen for the cars of tomorrow
- ^ Algae: Power Plant of the Future?
- ^ Reengineering Algae To Fuel The Hydrogen Economy
- ^ Melis A, Happe T (2001). "Hydrogen Production. Green Algae as a Source of Energy". Plant Physiol. 127 (3): 740–748. doi:10.1104/pp.010498. PMC 1540156. PMID 11706159.
- ^ Yang, Shihui; Guarnieri, Michael T; Smolinski, Sharon; Ghirardi, Maria; Pienkos, Philip T (2013). "De novo transcriptomic analysis of hydrogen production in the green alga Chlamydomonas moewusii through RNA-Seq". Biotechnology for Biofuels. 6 (1): 118. doi:10.1186/1754-6834-6-118. ISSN 1754-6834. PMC 3846465. PMID 23971877.
- ^ P. Häussinger, R. Lohmüller, A. M. Watson, "Hydrogen, 2. Production" in Ullmann's Encyclopedia of Industrial Chemistry, 2012, Wiley-VCH, Weinheim. doi:10.1002/14356007.o13_o03
- ^ Ogden, J.M. (1999). "Prospects for building a hydrogen energy infrastructure". Annual Review of Energy and the Environment. 24: 227–279. doi:10.1146/annurev.energy.24.1.227.
- ^ "Hydrogen Production: Natural Gas Reforming". Department of Energy. Retrieved 6 April 2017.
External links[]
- Anaerobic digestion
- Biodegradable waste management
- Biodegradation
- Biofuels
- Biotechnology products
- Fuel gas
- Fuels
- Hydrogen
- Hydrogen biology
- Hydrogen economy
- Hydrogen production
- Waste management