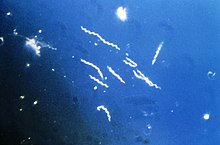
Borrelia burgdorferi
| Borrelia burgdorferi | |
|---|---|
| |
| Borrelia burgdorferi | |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Spirochaetes |
| Order: | Spirochaetales |
| Family: | Spirochaetaceae |
| Genus: | Borrelia |
| Species: | B. burgdorferi
|
| Binomial name | |
| Borrelia burgdorferi Johnson et al. 1984 emend. Baranton et al. 1992
| |
Borrelia burgdorferi sensu stricto is a bacterial species of the spirochete class in the genus Borrelia, and is one of the causative agents of Lyme disease in humans.[1][2] Along with a few similar genospecies, some of which also cause Lyme disease, it makes up the species complex of Borrelia burgdorferi sensu lato. The complex currently comprises 20 accepted and 3 proposed genospecies.[2] B. burgdorferi sensu stricto exists in North America and Eurasia and until 2016 was the only known cause of Lyme disease in North America.[3][4][2] Borrelia species are gram-negative.[5]
Microbiology[]
Borrelia burgdorferi is named after the researcher Willy Burgdorfer, who first isolated the bacterium in 1982.[6]
Morphology[]
B. burgdorferi resembles other spirochetes in that it has an outer membrane and inner membrane with a thin layer of peptidoglycan in between. However, the outer membrane lacks lipopolysaccharide. Its shape is a flat wave. It is about 0.3 μm wide and 5 to 20 μm in length.[7]
B. burgdorferi is a microaerobic, motile spirochete with seven to 11 bundled perisplasmic flagella set at each end that allow the bacterium to move in low- and high-viscosity media alike, which is related to its high virulence factor.[8]
Metabolism[]
B. burgdorferi is a slow-growing microaerophilic spirochete with a doubling time of 24 to 48 hours.[9]
Life cycle[]
B. burgdorferi circulates between Ixodes ticks and a vertebrate host in an enzootic cycle.[2] B. burgdorferi living in a tick is mainly acquired through blood meals from an infected, competent vertebrate host,[10] but rare cases of transovarial transmission exist.[11] Once a tick is infected, it will then transmit B. burgdorferi by feeding on another vertebrate to complete the cycle.[12] Ticks can transmit B. burgdorferi to humans, but humans are dead-end hosts, unlikely to continue the life cycle of the spirochete.[13] Nymphs molt into adult ticks, which usually feed on larger mammals that are not able to support the survival of B. burgdorferi.[14]
Disease[]
Lyme disease is a zoonotic, vector-borne disease transmitted by the Ixodes tick (also the vector for Babesia and Anaplasma). The infected nymphal tick transmits B. burgdorferi via its saliva to the human during its blood meal.[14]
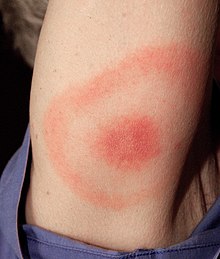
Clinical presentation of Lyme disease is best known for the characteristic bull's-eye rash (also known as erythema chronicum migrans) but can also include myocarditis, cardiomyopathy, arrythmia, arthritis, arthralgia, meningitis, neuropathies, and facial nerve palsy[15] depending on the stage of infection.
B. burgdorferi infections have been found in possible association with primary cutaneous B-cell lymphomas (PCBCLs),[16][17] where a review of the primary literature has, as of 2010, noted that most of the PCBCLs examined have been 'unresponsive' to antibiotics;[17]: 846 hence, as in the case of Chlamydophila psittaci association with ocular adnexal mucosa-associated lymphoid tissue (MALT) lymphoma, the working conclusion was that "if B. burgdorferi is truly associated with PCBCL, then there is wide geographic variability and other factors are probably involved".[17]: 846
Progression of the disease follows 3 stages.
Stage 1[]
Stage 1 is known as the Early Localized stage and occurs approximately 3 days - 1 month after inoculation. It affects the local area around the bite and is characterized by local swelling and / or a red "bull's-eye" rash (also known as erythema chronicum migrans) seen as an erythematous circle encircling a defined center that expands outward. It can get as large as 15 cm in diameter.[18]: 658 Once the rash starts to subside the first symptoms can manifest as "flu-like" symptoms. At this stage, antibiotics are most efficacious to prevent further growth and symptoms of the disease before the major symptoms manifest.[18]: 659
Stage 2[]
Stage 2 is known as the Early Disseminated stage and occurs weeks - months after infection if left untreated. The bacteria spreads via the blood through the body to affect the organs. It often presents with general symptoms such as fever, chills, fatigue, and lymphadenopathy as well as the organ-specific symptoms. It can affect the heart causing myocarditis and arrythmias such as atrioventricular blocks (which if significant enough may require the insertion of a pacemaker). It can affect the musculoskeletal system causing non-inflammatory transient arthritis and / or arthralgias. It can affect the nervous system manifesting as facial paralysis (Bell's palsy, classically bilateral), fatigue, and loss of memory.
Stage 3[]
Stage 3 is known as the Late Disseminated stage and occurs months - years after the initial infection. Effects of the 3rd stage include encephalitis or meningitis,[18] as well as migratory arthropathies (most commonly of the knee).[18]
Anaplasmosis and babesiosis are also common tick-borne pathogens carried by the Ixodes tick that infect humans similarly to Borrelia burgdorferi.[19] Consequently, it is possible for an Ixodes tick to coinfect a host with either two or all other diseases. When a host is coinfected, the combined effects of the diseases act synergistically, often proving to cause worse symptoms than a single infection alone[19] Coinfected humans tend to display a more severe manifestation of Lyme disease. In addition, they tend to acquire a wider range of secondary symptoms, such as influenza-like symptoms.[19] More studies and research must be done to determine the synergistic effect of co-infection and its effect on the human body.
Variation of severity[]
So far, there are three factors that may contribute to the severity of the clinical manifestation of Lyme Disease. The presence of ribosomal spacers, plasmids, and the outer surface protein C (OspC) are indicators of the severity of the infection.[20] Additionally, humans, themselves, vary in their response to the infection.[20] The variation in response leads to different clinical manifestations and different infections to different organs.
Additional information on Lyme disease and its cause[]
Antibiotic therapy resolves clinical symptoms, in most cases, during the early stages of infection. Persistent or relapsing symptoms can later develop in a subset of patients. The underlying mechanisms, pathogenesis, and treatment of PTLDS remain unknown. Therefore, it is of great importance to develop a vaccine that will prevent the incidence of serious tick-borne infections such as Lyme borreliosis. Most research efforts focus on the identification of either B. burgdorferi antigens or tick proteins that are required for the survival of spirochetes within ticks, in an attempt to interfere with pathogen transmission from ticks or infectivity in the hosts, thereby preventing Lyme disease.
Molecular pathogenesis[]
This section needs expansion with: an encyclopedic description of the pathogenesis that is sourced to good secondary literature. You can help by . (December 2015) |
After the pathogen is transmitted, it will acclimate to the mammalian conditions. Borrelia burgdorferi will change its glycoproteins and proteases on its plasma membrane to facilitate its dissemination throughout the blood.[20] While infecting, B. burgdorferi will express proteins that will interact with endothelial cells, platelets, chondrocytes, and the extracellular matrix.[20] This interaction inhibits proper function of the infected areas, leading to the pathological manifestations of Lyme disease. In response, the host will initiate an inflammatory response to attempt to remove the infection.[20]
Borrelia burgdorferi, also, expresses at least seven plasminogen binding proteins for interference of factor H at the activation level. This is part of a complement system evasion strategy that leads to downstream blocking of immune response.[21]
In addition, Borrelia burgdorferi has a strategy to directly inhibit the classical pathway of complement system. A borrelial lipoprotein BBK32, expressed on the surface of Borrelia burgdorferi, binds the initiating protease complex C1 of the classical pathway. More specifically, BBK32 interacts with C1r subunit of C1. C-terminal domain of the BBK32 protein mediates the binding. As a result, C1 is trapped in an inactive form. [22]
Genetics[]
B. burgdorferi (B31 strain) was the third microbial genome ever sequenced, following the sequencing of both Haemophilus influenzae and Mycoplasma genitalium in 1995. Its linear chromosome contains 910,725 base pairs and 853 genes.[23] The sequencing method used was whole genome shotgun. The sequencing project, published in Nature in 1997 and Molecular Microbiology in 2000, was conducted at The Institute for Genomic Research.[24] Overall, B. burgdorferi's genome oddly consists of one megabase chromosome and a variety of circular and linear plasmids ranging in size from 9 to 62 kilobases.[12] The megabase chromosome, unlike many other eubacteria, has no relation to either the bacteria's virulence or to the host-parasite interaction.[23] Some of the plasmids are necessary for the B. burgdorferi life cycle but not for propagation of the bacteria in culture.[12]
The genomic variations of B. burgdorferi contribute to varying degrees of infection and dissemination.[25] Each genomic group has varying antigens on its membrane receptor, which are specific to the infection of the host. One such membrane receptor is the surface protein OspC.[25] The OspC surface protein is shown to be a strong indicator of the identification of genomic classification and the degree of dissemination.[25] Varying number of OspC loci are indications and determinants for the variations of B. burgdorferi.[25] The surface protein is also on the forefront of current vaccine research for Lyme disease via Borrelia.[26]
Evolution[]
Genetically diverse B. burgdorferi strains, as defined by the sequence of ospC, are maintained within the Northeastern United States. Balancing selection may act upon ospC or a nearby sequence to maintain the genetic variety of B. burgdorferi.[27] Balancing selection is the process by which multiple versions of a gene are kept within the gene pool at unexpectedly high frequencies. Two major models that control the selection balance of B.burgdorferi is negative frequency-dependent selection and multiple-niche polymorphism[28]. These models may explain how B. burgdorferi have diversified, and how selection may have affected the distribution of the B. burgdorferi variants, or the variation of specific traits of the species, in certain environments.
Negative-frequency dependent selection[]
In negative frequency-dependent selection, rare and uncommon variants will have a selective advantage over variants that are very common in an environment.[28] For B. burgdorferi, low-frequency variants will be advantageous because potential hosts will be less likely to mount an immunological response to the variant-specific OspC outer protein.[28]
Multiple-niche polymorphism[]
Ecological niches are all of the variables in an environment, such as the resources, competitors, and responses, that contribute to the organism's fitness. Multiple-niche polymorphism states that diversity is maintained within a population due to the varying amount of possible niches and environments.[28] Therefore, the more various niches the more likelihood of polymophrism and diversity. For B. burgdorferi, varying vertebrae niches, such deer and mice, can affect the overall balancing selection for variants.[28]
See also[]
References[]
- ^ Radolf JD, Samuels DS, eds. (2021). Lyme Disease and Relapsing Fever Spirochetes: Genomics, Molecular Biology, Host Interactions, and Disease Pathogenesis. Caister Academic Press. ISBN 978-1-913652-61-6.
- ^ Jump up to: a b c d Wolcott, Katherine A.; Margos, Gabriele; Fingerle, Volker; Becker, Noémie S. (2021-09-01). "Host association of Borrelia burgdorferi sensu lato: A review". Ticks and Tick-borne Diseases. 12 (5): 101766. doi:10.1016/j.ttbdis.2021.101766. ISSN 1877-959X. PMID 34161868.
- ^ CDC (2016-02-08). "New Lyme-disease-causing bacteria species discovered". Centers for Disease Control and Prevention. Retrieved 2019-01-18.
- ^ Tilly, Kit; Rosa, Patricia A.; Stewart, Philip E. (2008). "Biology of Infection with Borrelia burgdorferi". Infectious Disease Clinics of North America. 22 (2): 217–234. doi:10.1016/j.idc.2007.12.013. PMC 2440571. PMID 18452798.
- ^ Samuels DS; Radolf, JD, eds. (2010). "Chapter 6, Structure, Function and Biogenesis of the Borrelia Cell Envelope". Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Caister Academic Press. ISBN 978-1-904455-58-5.
- ^ Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP (June 1982). "Lyme disease-a tick-borne spirochetosis?". Science. 216 (4552): 1317–9. Bibcode:1982Sci...216.1317B. doi:10.1126/science.7043737. PMID 7043737.
- ^ Motaleb MA, Liu J, Wooten RM (2015). "Spirochetal motility and chemotaxis in the natural enzootic cycle and development of Lyme disease". Current Opinion in Microbiology. 28: 106–13. doi:10.1016/j.mib.2015.09.006. PMC 4688064. PMID 26519910.
- ^ Motaleb, Mohammed; Corum, Linda; Bono, James; Elias, Abdallah; Rosa, Patricia; Samuels, D. Scott; Charon, Nyles (2000). "Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions". Proceedings of the National Academy of Sciences of the United States of America. 97 (20): 10899–10904. Bibcode:2000PNAS...9710899M. doi:10.1073/pnas.200221797. PMC 27121. PMID 10995478.
- ^ Zückert WR (2007). "Laboratory Maintenance of Borrelia burgdorferi". Laboratory maintenance of Borrelia burgdorferi. Current Protocols in Microbiology. Chapter 12. pp. Unit 12C.1. doi:10.1002/9780471729259.mc12c01s4. ISBN 978-0471729259. PMID 18770608.
- ^ Eisen, Rebecca J.; Eisen, Lars (April 2018). "The Blacklegged Tick, Ixodes Scapularis: An Increasing Public Health Concern". Trends in Parasitology. 34 (4): 295–6. doi:10.1016/j.pt.2017.12.006. PMC 5879012. PMID 29336985.
- ^ Rollend, Lindsay; Fish, Durland; Childs, James E. (2013-02-01). "Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations". Ticks and Tick-borne Diseases. 4 (1–2): 46–51. doi:10.1016/j.ttbdis.2012.06.008. ISSN 1877-959X. PMID 23238242.
- ^ Jump up to: a b c Brisson D, Drecktrah D, Eggers CH, Samuels DS (2012). "Genetics of B. burgdorferi". Annual Review of Genetics. 46: 515–36. doi:10.1146/annurev-genet-011112-112140. PMC 3856702. PMID 22974303.
- ^ Radolf JD, Caimano MJ, Stevenson B, Hu LT (2012). "Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes". Nature Reviews. Microbiology. 10 (2): 87–99. doi:10.1038/nrmicro2714. PMC 3313462. PMID 22230951.
- ^ Jump up to: a b Tilly K, Rosa PA, Stewart PE (2008). "Biology of infection with Borrelia burgdorferi". Infectious Disease Clinics of North America. 22 (2): 217–34, v. doi:10.1016/j.idc.2007.12.013. PMC 2440571. PMID 18452798.
- ^ "Signs and Symptoms, Lyme Disease". Centers For Disease Control. March 4, 2015. Retrieved 2015-07-16.
- ^ Guidoboni M, Ferreri AJ, Ponzoni M, Doglioni C, Dolcetti R (January 2006). "Infectious agents in mucosa-associated lymphoid tissue-type lymphomas: pathogenic role and therapeutic perspectives". Clinical Lymphoma & Myeloma. 6 (4): 289–300. doi:10.3816/CLM.2006.n.003. PMID 16507206.
- ^ Jump up to: a b c Chang, A. H.; Parsonnet, J. (2010). "Role of Bacteria in Oncogenesis". Clinical Microbiology Reviews. 23 (4): 837–857. doi:10.1128/CMR.00012-10. ISSN 0893-8512. PMC 2952975. PMID 20930075.
- ^ Jump up to: a b c d Tortora, Gerard J.; Funke, Berdell R.; Case, Christine L. (2013). Microbiology: An Introduction. United States of America: Pearson Education, Inc. pp. 658–659. ISBN 978-0-321-73360-3.
- ^ Jump up to: a b c Swanson, Stephen J.; Neitzel, David; Reed, Kurt D.; Belongia, Edward A. (2006-10-01). "Coinfections Acquired from Ixodes Ticks". Clinical Microbiology Reviews. 19 (4): 708–727. doi:10.1128/CMR.00011-06. ISSN 0893-8512. PMC 1592693. PMID 17041141.
- ^ Jump up to: a b c d e Weis, Janet (2011). "Critical Needs and Gaps in Understanding Prevention, Amelioration, and Resolution of Lyme and Other Tick-Borne Diseases: The Short-Term and Long-Term Outcomes: Workshop Report". The National Academies: 97–101.
- ^ Zipfel P., Hallström T., Riesbeck K. (2013). "Human complement control and complement evasion by pathogenic microbes – Tipping the balance". Molecular Immunology. 56 (3): 152–160. doi:10.1016/j.molimm.2013.05.222. PMID 23810413.CS1 maint: multiple names: authors list (link)
- ^ Garcia, B.L., Zhi, H., Wager, B., Höök, M. & Skare, J.T. 2016, "Borrelia burgdorferi BBK32 Inhibits the Classical Pathway by Blocking Activation of the C1 Complement Complex", PLoS Pathogens, vol. 12, no. 1
- ^ Jump up to: a b Fraser CM, Casjens S, Huang WM, et al. (December 1997). "Genomic sequence of a Lyme disease spirochaete, B. burgdorferi". Nature. 390 (6660): 580–6. Bibcode:1997Natur.390..580F. doi:10.1038/37551. PMID 9403685. S2CID 4388492.
- ^ Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, Rosa P, Lathigra R, Sutton G, Peterson J, Dodson RJ, Haft D, Hickey E, Gwinn M, White O, Fraser CM (2000). "A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete B. burgdorferi". Molecular Microbiology. 35 (3): 490–516. doi:10.1046/j.1365-2958.2000.01698.x. PMID 10672174.
- ^ Jump up to: a b c d Theisen, M.; Borre, M.; Mathiesen, M. J.; Mikkelsen, B.; Lebech, A. M.; Hansen, K. (1995-06-01). "Evolution of the Borrelia burgdorferi outer surface protein OspC". Journal of Bacteriology. 177 (11): 3036–3044. doi:10.1128/jb.177.11.3036-3044.1995. ISSN 0021-9193. PMC 176990. PMID 7768799.
- ^ Embers, Monica E.; Narasimhan, Sukanya (2013-02-12). "Vaccination against Lyme disease: past, present, and future". Frontiers in Cellular and Infection Microbiology. 3: 6. doi:10.3389/fcimb.2013.00006. ISSN 2235-2988. PMC 3569838. PMID 23407755.
- ^ Kurtenbach K, Hanincová K, Tsao JI, Margos G, Fish D, Ogden NH (2006). "Fundamental processes in the evolutionary ecology of Lyme borreliosis". Nature Reviews. Microbiology. 4 (9): 660–9. doi:10.1038/nrmicro1475. PMID 16894341. S2CID 10877654.
- ^ Jump up to: a b c d e Samuels, D. Scott (2010-01-01). Borrelia: Molecular Biology, Host Interaction and Pathogenesis. Horizon Scientific Press. ISBN 9781904455585.
Further reading[]
- Velázquez, Encarna, Peix, Álvaro & Gómez-Alonso, Alberto, 2011, "Microorganismos y cáncer: evidencias científicas y nuevas hipótesis", Cirugía Española, vol. 89, no. 3, pp. 136–144. ISSN 0009-739X; doi:10.1016/j.ciresp.2010.08.006; accessed 16 July 2015. English translation.
External links[]
| Wikispecies has information related to Borrelia burgdorferi. |
| Scholia has a topic profile for Borrelia burgdorferi. |
- Bacteria described in 1992
- Borrelia
- Lyme disease
- Suicide-inducing parasitism
