Etacrynic acid
 | |
| Clinical data | |
|---|---|
| Trade names | Edecrin |
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a682857 |
| Routes of administration | Oral, IV |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | > 98% |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.349 |
| Chemical and physical data | |
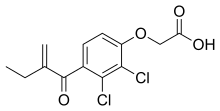
| Formula | C13H12Cl2O4 |
| Molar mass | 303.14 g·mol−1 |
| 3D model (JSmol) | |
Etacrynic acid (INN) or ethacrynic acid (USAN), trade name Edecrin, is a loop diuretic used to treat high blood pressure and the swelling caused by diseases like congestive heart failure, liver failure, and kidney failure.
Unlike the other loop diuretics, etacrynic acid is not a sulfonamide[1] and thus, its use is not contraindicated in those with sulfa allergies.
Ethacrynic acid is a phenoxyacetic acid derivative containing a ketone group and a methylene group. A cysteine adduct is formed with the methylene group and this is the active form.[citation needed]
Medical use[]
Ethacrynic acid is a diuretic that is used to treat edema when a stronger agent is required. It is available as a pill or injected form. The pill is used to treat edema associated with congestive heart failure, cirrhosis and renal disease, accumulation of liquid in the belly associated with cancer or edema, and management of hospitalized children with congenital heart disease or nephrotic syndrome. The injected form is used to rapidly remove water from the body when needed - for example in acute pulmonary edema - or when a person cannot take the medicine in pill form.[2]
Adverse effects[]
As a diuretic, ethacrynic acid can cause frequent urination, but this usually resolves after taking the drug for a few weeks.
Ethacrynic acid can also cause low potassium levels, which may manifest as muscle cramps or weakness. It has also been known to cause reversible or permanent hearing loss (ototoxicity)[3] and liver damage[4] when administered in extremely high dosages. On oral administration, it produces diarrhea; intestinal bleeding may occur at higher doses.
Mechanism of action[]
Ethacrynic acid acts by inhibiting NKCC2 in the thick ascending loop of Henle and the macula densa. Loss of potassium ions is less marked but chances of hypochloremic alkalosis are greater. The dose response curve of ethacrynic acid is steeper than that of furosemide and, in general, it is less manageable; dose range is 50-150mg.
Ethacrynic acid and its glutathione-adduct are potent inhibitors of glutathione S-transferase family members, which are enzymes involved in xenobiotic metabolism. This family of enzymes has recently been shown to have a high rate of genetic variability.
United States Price History[]
The ethacrynic acid tablet market had U.S. sales of approximately $17 million for the 12 months ending April 2020 according to IQVIA.[5]
National Average Drug Acquisition Cost data from the Centers for Medicare and Medicaid Services[6] shows that the average price paid by retail pharmacies for an Edecrin 25 MG tablet was $5.24 as 11/28/13 by Bausch Health US, LLC, formerly Valeant Pharmaceuticals. The price from Bausch Health US, LLC increased to $21.72 per tablet as of 5/18/2016.
In 2015, Valeant was involved in a number of controversies surrounding drug price hikes and the use of a specialty pharmacy for the distribution of its drugs.[7]
As of 9/19/2018, the price for the generic equivalent was $10.01 per tablet from West-ward Pharmaceuticals Corp., Edenbridge Pharmaceuticals, LLC and Oceanside Pharmaceuticals. As of 7/22/2020 the price decreased to $3.45 with availability from additional generic manufacturers. As of 2/17/2021 the average price paid by pharmacies was $5.69 per 25 MG tablet with 10 generic manufacturers.
| Company Name | Effective_Date | NADAC_Per_Unit |
| West-ward Pharmaceuticals Corp. | 9/19/2018 | 10.01 |
| Edenbridge Pharmaceuticals, Llc | 9/19/2018 | 10.01 |
| Oceanside Pharmaceuticals | 9/19/2018 | 10.01 |
| Alvogen, Inc. | 10/10/2018 | 10.01 |
| Par Pharmaceutical, Inc. | 11/14/2018 | 10.01 |
| Amneal Pharmaceuticals Ny Llc | 2/27/2019 | 10.01 |
| West-ward Pharmaceuticals Corp. | 4/22/2020 | 9.81 |
| Edenbridge Pharmaceuticals, Llc | 4/22/2020 | 9.81 |
| Alvogen, Inc. | 4/22/2020 | 9.81 |
| Par Pharmaceutical, Inc. | 4/22/2020 | 9.81 |
| Sciegen Pharmaceuticals Inc | 4/22/2020 | 9.81 |
| Lupin Pharmaceuticals, Inc. | 4/22/2020 | 9.81 |
| Oceanside Pharmaceuticals | 4/22/2020 | 9.81 |
| Amneal Pharmaceuticals Ny Llc | 4/22/2020 | 9.81 |
| West-ward Pharmaceuticals Corp. | 5/20/2020 | 5.76 |
| Edenbridge Pharmaceuticals, Llc | 5/20/2020 | 5.76 |
| Alvogen, Inc. | 5/20/2020 | 5.76 |
| Par Pharmaceutical, Inc. | 5/20/2020 | 5.76 |
| Sciegen Pharmaceuticals Inc | 5/20/2020 | 5.76 |
| Lupin Pharmaceuticals, Inc. | 5/20/2020 | 5.76 |
| Oceanside Pharmaceuticals | 5/20/2020 | 5.76 |
| Amneal Pharmaceuticals Ny Llc | 5/20/2020 | 5.76 |
| West-ward Pharmaceuticals Corp. | 7/22/2020 | 3.45 |
| Edenbridge Pharmaceuticals, Llc | 7/22/2020 | 3.45 |
| Alvogen, Inc. | 7/22/2020 | 3.45 |
| Par Pharmaceutical, Inc. | 7/22/2020 | 3.45 |
| Sciegen Pharmaceuticals Inc | 7/22/2020 | 3.45 |
| Lupin Pharmaceuticals, Inc. | 7/22/2020 | 3.45 |
| Oceanside Pharmaceuticals | 7/22/2020 | 3.45 |
| Amneal Pharmaceuticals Ny Llc | 7/22/2020 | 3.45 |
| Trupharma Llc | 7/29/2020 | 3.45 |
| West-ward Pharmaceuticals Corp. | 8/19/2020 | 5.08 |
| Edenbridge Pharmaceuticals, Llc | 8/19/2020 | 5.08 |
| Alvogen, Inc. | 8/19/2020 | 5.08 |
| Par Pharmaceutical, Inc. | 8/19/2020 | 5.08 |
| Sciegen Pharmaceuticals Inc | 8/19/2020 | 5.08 |
| Trupharma Llc | 8/19/2020 | 5.08 |
| Lupin Pharmaceuticals, Inc. | 8/19/2020 | 5.08 |
| Oceanside Pharmaceuticals | 8/19/2020 | 5.08 |
| Amneal Pharmaceuticals Ny Llc | 8/19/2020 | 5.08 |
| Upsher-smith Laboratories, Llc | 9/16/2020 | 5.08 |
| West-ward Pharmaceuticals Corp. | 2/17/2021 | 5.69 |
| Upsher-smith Laboratories, Llc | 2/17/2021 | 5.69 |
| Edenbridge Pharmaceuticals, Llc | 2/17/2021 | 5.69 |
| Alvogen, Inc. | 2/17/2021 | 5.69 |
| Par Pharmaceutical, Inc. | 2/17/2021 | 5.69 |
| Sciegen Pharmaceuticals Inc | 2/17/2021 | 5.69 |
| Trupharma Llc | 2/17/2021 | 5.69 |
| Lupin Pharmaceuticals, Inc. | 2/17/2021 | 5.69 |
| Oceanside Pharmaceuticals | 2/17/2021 | 5.69 |
| Amneal Pharmaceuticals Ny Llc | 2/17/2021 | 5.69 |
Price Fixing Investigation[]
It is known whether this apparent price fixing is actively being investigated, but the United States Department of Justice Antitrust Division Spring Update 2021 notes:
"The Division remains committed to rooting out illegal conduct that corrupts critical healthcare markets. That work is more important now than ever before.
In recent years, the Division has uncovered price-fixing, bid-rigging, and customer-allocation schemes in one of the most important markets for the health and wallets of American consumers: the generic drug industry. Indeed, nearly 90% of all prescriptions in the United States are filled with generic drugs."[8]
References[]
- ^ Somberg JC, Molnar J (January 2009). "The pleiotropic effects of ethacrynic acid". American Journal of Therapeutics. 16 (1): 102–4. doi:10.1097/MJT.0b013e3181961264. PMID 19142157.
- ^ Merck and FDA Etacrynic acid Label. Last updated February 2005 per FDA site for NDA 016093 injected form and FDA site for NDA 016092 oral form per index here, each accessed January 16, 2016
- ^ Bosher SK (1980). "The nature of the ototoxic actions of ethacrynic acid upon the mammalian endolymph system. I. Functional aspects". Acta Oto-Laryngologica. 89 (5–6): 407–18. doi:10.3109/00016488009127156. PMID 7446061.
- ^ Datey KK, Deshmukh SN, Dalvi CP, Purandare NM (July 1967). "Hepatocellular damage with ethacrynic acid". British Medical Journal. 3 (5558): 152–3. doi:10.1136/bmj.3.5558.152. PMC 1842848. PMID 6028103.
- ^ "Upsher-Smith Launches Ethacrynic Acid Tablets, USP - Upsher-Smith". Upsher-Smith Laboratories, LLC. 2020-06-23. Retrieved 2021-03-14.
- ^ "NADAC (National Average Drug Acquisition Cost) | Data.Medicaid.gov". data.medicaid.gov. Retrieved 2021-03-14.
- ^ "Valeant's price-hike strategy goes far beyond two high-profile increases". FiercePharma. Retrieved 2021-03-14.
- ^ "Generic Drugs Investigation Targets Anticompetitive Schemes". 10 March 2021.
- Disulfiram-like drugs
- Loop diuretics
- Piceol ethers
- Acetic acids
- Chloroarenes
- Carbonic anhydrase inhibitors
- World Anti-Doping Agency prohibited substances