Finerenone
 | |
| Clinical data | |
|---|---|
| Trade names | Kerendia |
| Other names | BAY 94-8862 |
| License data |
|
| Routes of administration | By mouth |
| Drug class | Potassium-sparing diuretic |
| ATC code |
|
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.614 |
| Chemical and physical data | |
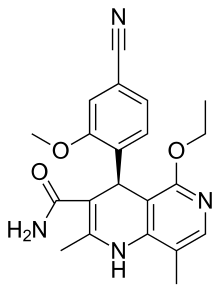
| Formula | C21H22N4O3 |
| Molar mass | 378.432 g·mol−1 |
| 3D model (JSmol) | |
Finerenone, sold under the brand name Kerendia, is a medication used to reduce the risk of kidney function decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure in adults with chronic kidney disease associated with type 2 diabetes.[2] Finerenone is a non-steroidal mineralocorticoid receptor antagonist (MRA).[1]
Common side effects include hyperkalemia (high levels of potassium), hypotension (low blood pressure), and hyponatremia (low levels of sodium).[2]
Finerenone was approved for medical use in the United States in July 2021.[2][3]
Medical uses[]
Finerenone is indicated to reduce the risk of kidney function decline, kidney failure, cardiovascular death, non-fatal heart attacks, and hospitalization for heart failure in adults with chronic kidney disease associated with type 2 diabetes.[2]
Pharmacology[]
Finerenone has less relative affinity to other steroid hormone receptors than currently available aldosterone antagonists such as eplerenone and spironolactone, which should result in fewer adverse effects like gynaecomastia, impotence, and low libido.[4][5]
Finerenone blocks mineralocorticoid receptors, which makes it a potassium-sparing diuretic.
This table compares inhibitory (blocking) concentrations (IC50, unit: nM) of three antimineralocorticoids. Mineralocorticoid receptor inhibition is responsible for the desired action of the drugs, whereas inhibition of the other receptors potentially leads to side effects. Lower values mean stronger inhibition.[6][full citation needed]
| Spironolactone | Eplerenone | Finerenone | |
|---|---|---|---|
| Mineralocorticoid receptor | 24 | 990 | 18 |
| Glucocorticoid receptor | 2400 | 22,000 | >10,000 |
| Androgen receptor | 77 | 21,200 | >10,000 |
| Progesterone receptor | 740 | 31,200 | >10,000 |
The above-listed drugs have insignificant affinity for the estrogen receptor.[citation needed]
Finerenone acts as an antagonist to mineralocorticoid receptors harboring the S810L mutation, unlike other traditional MR inhibitors such as spironolactone and eplerenone that incidentally act as agonists.[7]
History[]
The efficacy of finerenone to improve kidney and heart outcomes was evaluated in a randomized, multicenter, double-blind, placebo-controlled study in adults with chronic kidney disease associated with type 2 diabetes.[2] In this study, 5,674 participants were randomly assigned to receive either finerenone or a placebo.[2]
The study compared the two groups for the number of participants whose disease progressed to a composite (or combined) endpoint that included at least a 40% reduction in kidney function, progression to kidney failure, or kidney death.[2] Results showed that 504 of the 2,833 participants who received finerenone had at least one of the events in the composite endpoint compared to 600 of the 2,841 participants who received a placebo.[2]
The U.S. Food and Drug Administration (FDA) granted the application for finerenone priority review and fast track designations.[2] The FDA granted the approval of Kerendia to Bayer Healthcare.[2]
Research[]
In the Phase II ARTS-DN study, finerenone dose-dependently reduced urine albumin to creatinine ratio in patients with diabetic kidney disease.[8] Based on these findings, finerenone is being studied in the large Phase III FIDELIO and FIGARO outcome studies designed to assess whether finerenone reduces risk of CKD progression and adverse cardiovascular events in patients with Chronic Kidney Disease and Type 2 Diabetes. These trials have enrolled more than 13,000 patients with primary completion of FIDELIO anticipated in 2020 and FIGARO IN 2021.[9][full citation needed][10][full citation needed]
References[]
- ^ Jump up to: a b "Kerendia- finerenone tablet, film coated". DailyMed. Retrieved 20 August 2021.
- ^ Jump up to: a b c d e f g h i j k "FDA Approves Drug for Chronic Kidney Disease". U.S. Food and Drug Administration (FDA). 9 July 2021. Retrieved 9 July 2021.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ "Bayer's Kerendia (finerenone) Receives U.S. FDA Approval for Treatment of Patients with Chronic Kidney Disease Associated with Type 2 Diabetes" (Press release). Bayer. 9 July 2021. Retrieved 9 July 2021 – via Business Wire.
- ^ Ruilope LM, Tamargo J (April 2017). "Renin-angiotensin system blockade: Finerenone". Nephrologie & Therapeutique. 13 Suppl 1: S47–S53. doi:10.1016/j.nephro.2017.02.003. PMID 28577743.
- ^ Pitt B, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, et al. (February 2015). "Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease" (PDF). European Journal of Heart Failure. 17 (2): 224–32. doi:10.1002/ejhf.218. hdl:2027.42/110733. PMID 25678098. S2CID 205781715.
- ^ Schubert-Zsilavecz M, Wurglics M (Fall 2015). "Finerenone". Neue Arzneimittel.
- ^ Amazit L, Le Billan F, Kolkhof P, Lamribet K, Viengchareun S, Fay MR, et al. (September 2015). "Finerenone Impedes Aldosterone-dependent Nuclear Import of the Mineralocorticoid Receptor and Prevents Genomic Recruitment of Steroid Receptor Coactivator-1". The Journal of Biological Chemistry. 290 (36): 21876–89. doi:10.1074/jbc.M115.657957. PMC 4571943. PMID 26203193.
- ^ Bakris GL, Agarwal R, Chan JC, Cooper ME, Gansevoort RT, Haller H, et al. (September 2015). "Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy: A Randomized Clinical Trial". JAMA. 314 (9): 884–94. doi:10.1001/jama.2015.10081. PMID 26325557.
- ^ Clinical trial number NCT02540993 for "Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease (FIDELIO-DKD)" at ClinicalTrials.gov
- ^ Clinical trial number NCT02545049 for "Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and the Clinical Diagnosis of Diabetic Kidney Disease (FIGARO-DKD)" at ClinicalTrials.gov
Further reading[]
- Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. (December 2020). "Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes". N Engl J Med. 383 (23): 2219–2229. doi:10.1056/NEJMoa2025845. PMID 33264825.
External links[]
- "Finerenone". Drug Information Portal. U.S. National Library of Medicine.
- Drugs not assigned an ATC code
- Antimineralocorticoids
- Carboxamides
- Phenol ethers
- Nitriles
- Naphthyridines
