Mercury(II) nitrate
| |
| Names | |
|---|---|
| IUPAC names
Mercury dinitrate
Mercury(II) nitrate | |
| Other names
Mercuric nitrate
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.030.126 |
| EC Number |
|
PubChem CID
|
|
| RTECS number |
|
| UNII | |
| UN number | 1625 |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
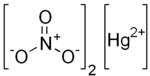
| Hg(NO3)2 | |
| Molar mass | 324.60 g/mol (anhydrous) |
| Appearance | colorless crystals or white powder |
| Odor | sharp |
| Density | 4.3 g/cm3 (monohydrate) |
| Melting point | 79 °C (174 °F; 352 K) (monohydrate) |
| soluble | |
| Solubility | soluble in nitric acid, acetone, ammonia insoluble in alcohol |
| −74.0·10−6 cm3/mol | |
| Hazards | |
| Safety data sheet | ICSC 0980 |
| GHS labelling: | |
   
| |
Signal word
|
Danger |
| H272, H300, H310, H330, H373, H410 | |
| NFPA 704 (fire diamond) | 
3
0
1 OX |
| Flash point | Non-flammable |
| Related compounds | |
Other anions
|
Mercury(II) sulfate Mercury(II) chloride |
Other cations
|
Zinc nitrate Cadmium nitrate |
Related compounds
|
Mercury(I) nitrate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mercury(II) nitrate is an inorganic compound with the formula Hg(NO3)2.xH2O. These colorless or white soluble crystalline salts are occasionally used as a reagent. It is made by treating mercury with hot concentrated nitric acid. Neither anhydrous nor monohydrate has been confirmed by X-ray crystallography.[1] The anhydrous material is more widely used.
Uses[]
Mercuric nitrate has been used in mercuration of ketones.[2]
Health information[]
Mercury compounds are highly toxic.
See also[]
- The Hatter
- Mercury poisoning
- Gilding
References[]
- ^ . doi:10.1002/zaac.200500344. Cite journal requires
|journal=(help); Missing or empty|title=(help) - ^ Morton, Avery A.; Penner, Hellmut P. (1951). "Mercuration of Ketones and Some Other Compounds with Mercuric Nitrate". Journal of the American Chemical Society. 73 (7): 3300–3304. doi:10.1021/ja01151a091.
External links[]
- ATSDR - Toxic Substances Portal - Mercury (11/14/2013)
- ATSDR - Public Health Statement: Mercury (11/14/2013)
- ATSDR - ALERT! Patterns of Metallic Mercury Exposure, 6/26/97 (link not traceable 11/14/2013)
- ATSDR - Medical Management Guidelines for Mercury (11/14/2013)
- ATSDR - Toxicological Profile: Mercury (11/14/2013)
- Safety data (MSDS) (link not traceable 11/14/2013)
- Mercuric Nitrate (ICSC)
- Mercury
- Mercury Information Packages
- How to Make Good Mercury Electrical Connections, Popular Science monthly, February 1919, Unnumbered page, Scanned by Google Books: https://books.google.com/books?id=7igDAAAAMBAJ&pg=PT14
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO 3)− 4 |
RONO2 | NO− 3 NH4NO3 |
HOONO2 | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)2 Fe(NO3)3 |
Co(NO3)2 Co(NO3)3 |
Ni(NO3)2 | CuNO3 Cu(NO3)2 |
Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | BrNO3 | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru(NO3)3 | Rh(NO3)3 | Pd(NO3)2 |
AgNO3 |
Cd(NO3)2 | In(NO3)3 | Sn(NO3)4 | Sb(NO3)3 | Te | INO3 | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf(NO3)4 | Ta | W | Re | Os | Ir | Au(NO3)3 | Hg2(NO3)2 Hg(NO3)2 |
Tl(NO3)3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po(NO3)4 | At | Rn | ||
| Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3 Ce(NO3)4 |
Pr(NO3)3 | Nd(NO3)3 | Pm(NO3)3 | Sm(NO3)3 | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy(NO3)3 | Ho(NO3)3 | Er(NO3)3 | Tm(NO3)3 | Yb(NO3)3 | Lu(NO3)3 | |||
| Ac(NO3)3 | Th(NO3)4 | UO2(NO3)2 | Np(NO3)4 | Pu(NO3)4 | Am(NO3)3 | Cm(NO3)3 | Bk | Cf | Es | Fm | Md | No | Lr | ||||
Categories:
- Mercury(II) compounds
- Nitrates
- Oxidizing agents