Phosphorus heptabromide
| |

| |
| Names | |
|---|---|
| Other names
Tetrabromophosphonium tribromide
Perbromophosphonium tribromide Phosphorus heptabromide | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Br7P | |
| Molar mass | 590.302 g·mol−1 |
| Structure[1] | |
| orthorhombic | |
| Pnma, No. 64 | |
a = 9.35 Å, b = 7.94 Å, c = 14.69 Å
| |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Phosphorus heptabromide is an inorganic compound with the formula PBr7. It is one of the phosphorus bromides. At normal conditions, it forms red prismatic crystals. PBr7 can be prepared by the sublimation of a mixture of phosphorus pentabromide and bromine.[2]
- PBr5 + Br2 → PBr7
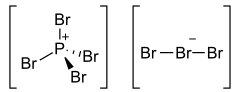
The structure consists of a PBr4+ cation paired with a tribromide (Br3–) anion, and the tribromide is non-symmetric.[1]
See also[]
References[]
- ^ a b Breneman, G. L.; Willett, R. D. (1967). "The crystal structure of phosphorus heptabromide, PBr7". Acta Crystallographica. 23 (3): 467–471. doi:10.1107/S0365110X67002981.
- ^ T. E. (Thomas Edward) Thorpe. A dictionary of applied chemistry (Volume 4)
Categories:
- Bromides
- Phosphorus halides
- Polyhalides
- Phosphonium compounds
- Inorganic compound stubs