Silicon disulfide

| |

| |
| |
| Names | |
|---|---|
| IUPAC name
silicon(IV) sulfide
| |
| Other names
silicon disulfide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.033.935 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| SiS2 | |
| Molar mass | 92.218 g/mol |
| Appearance | White (samples are sometimes grey or brown) needles. Rotten egg smell in moist air. |
| Density | 1.853 g/cm3 |
| Melting point | 1,090 °C (1,990 °F; 1,360 K) sublimes |
| Decomposes | |
| Structure | |
| Orthorhombic, oI12 | |
| Ibam, No.72[1] | |
| Tetrahedral | |
| Hazards | |
| NFPA 704 (fire diamond) | 
2
2
3 |
| Related compounds | |
Other anions
|
silicon dioxide |
Other cations
|
carbon disulfide germanium disulfide tin(IV) sulfide lead(IV) sulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
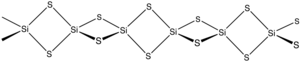
Silicon disulfide is the inorganic compound with the formula SiS2. Like silicon dioxide, this material is polymeric, but it adopts a 1-dimensional structure quite different from the usual forms of SiO2.
Synthesis, structure, and properties[]
The material is formed by heating silicon and sulfur or by the exchange reaction between SiO2 and Al2S3. The material consists of chains of edge-shared tetrahedra, Si(μ-S)2Si(μS)2, etc.[2]
Like other silicon sulfur-compounds (e.g., bis(trimethylsilyl)sulfide) SiS2 hydrolyzes readily to release H2S. In liquid ammonia it is reported to form the imide Si(NH)2 and NH4SH,[3] but a recent report has identified crystalline (NH4)2[SiS3(NH3)]·2NH3 as a product which contains the tetrahedral thiosilicate anion, SiS3(NH3).[4]
Reaction with ethanol gives the alkoxide tetraethyl orthosilicate and H2S.[3] With bulky tert-butanol, alcoholysis gives tris(tert-butoxy)silanethiol:[5]
- 3 (CH3)3COH + SiS2 → [(CH3)3CO]3SiSH + H2S
Reaction with sodium sulfide, magnesium sulfide and aluminum sulfide give thiosilicates.[3]
SiS2 is claimed to occur in certain interstellar objects.[6]
References[]
- ^ Weiss, A.; Weiss, A. (1954). "Über Siliciumchalkogenide. VI. Zur Kenntnis der faserigen Siliciumdioxyd-Modifikation". Zeitschrift für Anorganische und Allgemeine Chemie. 276 (1–2): 95–112. doi:10.1002/zaac.19542760110.
- ^ Holleman, A. F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 0-12-352651-5. A printing error in this book states that rSiSi is 214 picometers, when in fact that distance describes rSiS.
- ^ a b c Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. p. 359. ISBN 978-0-08-022057-4.
- ^ Meier, Martin; Korber, Nikolaus (2009). "The first thiosilicate from solution: synthesis and crystal structure of (NH4)2[SiS3(NH3)]·2NH3". Dalton Transactions (9): 1506–1508. doi:10.1039/b818856d. ISSN 1477-9226. PMID 19421590.
- ^ R. Piękoś, W. Wojnowski (1962). "Untersuchungen über die Alkoholyse des SiS2. II. Darstellung von Trialkoxysilanthiolen und Tetraalkoxycyclodisilthianen aus den tertiären Alkoholen". Z. Anorg. Allg. Chem. 318: 212–216. doi:10.1002/zaac.19623180310.
- ^ Goebel, J. H. (1993). "SiS2 in Circumstellar Shells" (PDF). Astronomy and Astrophysics. 278 (1): 226–230. Bibcode:1993A&A...278..226G.
- Inorganic silicon compounds
- Sulfides
- Inorganic polymers
- Dichalcogenides
- Inorganic compound stubs