Substituted tryptamine

Substituted tryptamines, or serotonin analogues, are organic compounds which may be thought of as being derived from tryptamine itself. The molecular structures of all tryptamines contain an indole ring, joined to an amino (NH2) group via an ethyl (−CH2–CH2−) sidechain. In substituted tryptamines, the indole ring, sidechain, and/or amino group are modified by substituting another group for one of the hydrogen (H) atoms.
Well-known tryptamines include serotonin, an important neurotransmitter, and melatonin, a hormone involved in regulating the sleep-wake cycle. Tryptamine alkaloids are found in fungi, plants and animals; and sometimes used by humans for the neurological or psychotropic effects of the substance. Prominent examples of tryptamine alkaloids include psilocybin (from "Psilocybin mushrooms") and DMT. In South America, dimethyltryptamine is obtained from numerous plant sources, like chacruna, and it is often used in ayahuasca brews. Many synthetic tryptamines have also been made, including the migraine drug sumatriptan, and psychedelic drugs.
The tryptamine structure, in particular its indole ring, may be part of the structure of some more complex compounds, for example: LSD, ibogaine, mitragynine and yohimbine. A thorough investigation of dozens of tryptamine compounds was published by Ann and Alexander Shulgin under the title TiHKAL.
List of substituted tryptamines[]
This list is incomplete; you can help by . (October 2021) |
| Chemical structure | Short Name | Origin | Ring Substitution | RN1 | RN2 | Full Name | CAS Number |
|---|---|---|---|---|---|---|---|
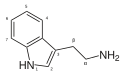 |
Tryptamine | Animals, plants, fungi | H | H | H | 3-(2-aminoethyl)indole / 2-(1H-indol-3-yl)ethanamine | 61-54-1 |
 |
NMT | Plants | H | H | CH3 | N-methyltryptamine | 61-49-4 |
 |
2-HO-NMT | Plants | 2-OH | H | CH3 | 2-hydroxy-N-methyltryptamine | 106987-89-7 |
 |
5-MeO-NMT | Plants | 5-OCH3 | H | CH3 | 5-methoxy-N-methyltryptamine | 2009-03-2 |
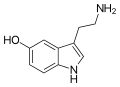 |
Serotonin | Animals, plants | 5-OH | H | H | 5-hydroxytryptamine | 50-67-9 |
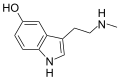 |
Nω-Methylserotonin (norbufotenin) | Plants | 5-OH | H | CH3 | 5-hydroxy-N-methyltryptamine | 1134-01-6 |
 |
Bufotenin | Animals, plants, fungi | 5-OH | CH3 | CH3 | 5-hydroxy-N,N-dimethyltryptamine | 487-93-4 |
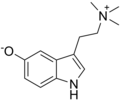 |
Bufotenidine | Amphibians | 5-O− | (CH3)3 | 3-[2-(trimethylazaniumyl)ethyl]-1H-indol-5-olate | 487-91-2 | |
 |
Melatonin | Animals, plants, microbes | 5-OCH3 | H | O=C-CH3 | 5-methoxy-N-acetyltryptamine | 73-31-4 |
 |
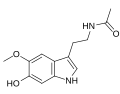
N-Acetylserotonin | Animals | 5-OH | H | O=C-CH3 | 5-hydroxy-N-acetyltryptamine | 1210-83-9 |
 |
6-Hydroxymelatonin | Animals | 5-OCH3, 6-OH | H | O=C-CH3 | N-[2-(6-Hydroxy-5-methoxy-1H-indol-3-yl)ethyl]acetamide | 2208-41-5 |
 |
4-HO-NMT | Fungi | 4-OH | H | CH3 | 4-hydroxy-N-methyltryptamine | 28363-70-4 |
 |
Psilocin | Fungi | 4-OH | CH3 | CH3 | 4-hydroxy-N,N-dimethyltryptamine | 520-53-6 |
 |
Norbaeocystin | Fungi | 4-OPO3H2 | H | H | 4-phosphoryloxy-tryptamine | 21420-59-7 |
 |
Baeocystin | Fungi | 4-OPO3H2 | H | CH3 | 4-phosphoryloxy-N-methyl-tryptamine | 21420-58-6 |
 |
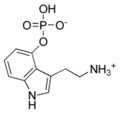
Psilocybin | Fungi | 4-OPO3H2 | CH3 | CH3 | 4-phosphoryloxy-N,N-dimethyltryptamine | 520-52-5 |
 |
Aeruginascin | Fungi | 4-OPO3H2 | (CH3)3 | [3-[2-(trimethylazaniumyl)ethyl]-1H-indol-4-yl] hydrogen phosphate | 114264-95-8 | |
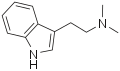 |
DMT | Animals, plants | H | CH3 | CH3 | N,N-dimethyltryptamine | 61-50-7 |
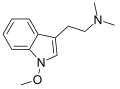 |
Lespedamine | Plants | 1-OCH3 | CH3 | CH3 | 1-methoxy-N,N-dimethyltryptamine | 4335-93-7 |
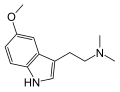 |
5-MeO-DMT | Animals, plants | 5-OCH3 | CH3 | CH3 | 5-methoxy-N,N-dimethyltryptamine | 1019-45-0 |
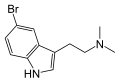 |
5-Bromo-DMT | Marine sponges, invertebrates | 5-Br | CH3 | CH3 | 5-bromo-N,N-dimethyltryptamine | 17274-65-6 |
 |
6-Bromotryptamine | Marine invertebrates | 6-Br | H | H | 6-bromotryptamine | 96624-18-9 |
 |
5,6-Dibromotryptamine | Marine invertebrates | 5,6-Br | H | H | 5,6-dibromotryptamine | |
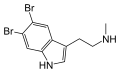 |
5,6-Dibromo-N-methyltryptamine | Marine invertebrates | 5,6-Br | H | CH3 | 5,6-dibromo-N-methyltryptamine | |
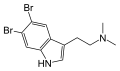 |
5,6-Dibromo-DMT | Marine sponges, invertebrates | 5,6-Br | CH3 | CH3 | 5,6-dibromo-N,N-dimethyltryptamine | 72853-80-6 |
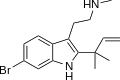 |
Desformylflustrabromine | Marine invertebrates | 2-(α,α-dimethylallyl), 6-Br | H | CH3 | 2-[6-bromo-2-(2-methylbut-3-en-2-yl)-1H-indol-3-yl]-N-methylethanamine | 474657-72-2 |
 |
Convolutindole A | Marine invertebrates | 2,4,6-Br, 1,7-OCH3 | CH3 | CH3 | 1,7-dimethoxy-2,4,6-tribromo-N,N-dimethyltryptamine | 443356-86-3 |
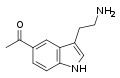 |
Acetryptine | artificial | 5-COCH3 | H | H | 5-Acetyltryptamine | 3551-18-6 |
 |
5-BT | artificial | 5-OCH2C6H5 | H | H | 5-Benzyloxytryptamine | 20776-45-8 |
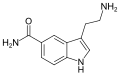 |
5-CT | artificial | 5-CONH2 | H | H | 5-Carboxamidotryptamine | 74885-09-9 |
 |
5-(Nonyloxy)tryptamine | artificial | 5-O(CH2)8CH3 | H | H | 5-nonyloxytryptamine | 157798-12-4 |
 |
2-Methyl-5-hydroxytryptamine | artificial | 2-CH3, 5-OH | H | H | 3-(2-aminoethyl)-2-methyl-1H-indol-5-ol | 78263-90-8 |
 |
NET | artificial | H | H | CH2CH3 | N-ethyltryptamine | 61-53-0 |
 |
NiPT | artificial | H | H | CH(CH3)2 | N-isopropyltryptamine | 14121-10-9 |
 |
NcPT | artificial | H | H | C3H5 | N-cyclopropyltryptamine | |
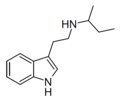 |
NSBT | artificial | H | H | CH(CH3)CH2CH3 | N-sec-butyltryptamine | |
 |
NTBT | artificial | H | H | C(CH3)3 | N-[2-(1H-indol-3-yl)ethyl]-2-methylpropan-2-amine | |
 |
5-MT-NBOMe | artificial | 5-OCH3 | H | CH2C6H4(o-OCH3) | 5-methoxy-N-(ortho-methoxybenzyl)tryptamine | 1335331-37-7 |
 |
5-MT-NB3OMe [1] | artificial | 5-OCH3 | H | CH2C6H4(m-OCH3) | 5-methoxy-N-(meta-methoxybenzyl)tryptamine | 1648553-42-7 |
 |
5-MeO-NBpBrT | artificial | 5-OCH3 | H | CH2C6H4(p-Br) | N-(4-Bromobenzyl)-2-(5-methoxy-1H-indol-3-yl)ethanamine | 155639-13-7 |
| Idalopirdine | artificial | 6-F | H | CH2C6H4(m-OCH2CF2CF2H) | 2-(6-Fluoro-1H-indol-3-yl)-N-(3-(2,2,3,3-tetrafluoropropoxy)benzyl)ethanamine | 467459-31-0 | |
 |
Pyr-T | artificial | H | (CH2)4 | 3-[2-(Pyrrolidin-1-yl)ethyl]-1H-indole | 14008-96-9 | |
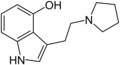 |
4-HO-pyr-T | artificial | 4-OH | (CH2)4 | 3-[2-(Pyrrolidin-1-yl)ethyl]-1H-indol-4-ol | 63097-26-7 | |
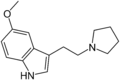 |
5-MeO-pyr-T | artificial | 5-OCH3 | (CH2)4 | 5-Methoxy-3-[2-(pyrrolidin-1-yl)ethyl]-1H-indole | 3949-14-2 | |
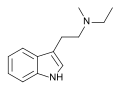 |
MET | artificial | H | CH3 | CH2CH3 | N-Methyl-N-ethyltryptamine | 5599-69-9 |
 |
MPT | artificial | H | CH3 | CH2CH2CH3 | N-Methyl-N-propyltryptamine | 850032-72-3 |
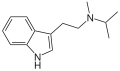 |
MiPT | artificial | H | CH3 | CH(CH3)2 | N-Methyl-N-isopropyltryptamine | 96096-52-5 |
 |
McPT | artificial | H | CH3 | C3H5 | N-Methyl-N-cyclopropyltryptamine | 1373918-63-8 |
 |
EcPT | artificial | H | CH2CH3 | C3H5 | N-ethyl-N-cyclopropyltryptamine | |
 |
PcPT | artificial | H | CH2CH2CH3 | C3H5 | N-propyl-N-cyclopropyltryptamine | |
 |
iPcPT | artificial | H | CH(CH3)2 | C3H5 | N-isopropyl-N-cyclopropyltryptamine | |
 |
DcPT | artificial | H | C3H5 | C3H5 | N,N-dicyclopropyltryptamine | 1373918-62-7 |
 |
MBT | artificial | H | CH3 | (CH2)3CH3 | N-Methyl-N-butyltryptamine | 848130-12-1 |
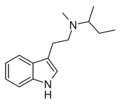 |
MSBT | artificial | H | CH3 | CH(CH3)CH2CH3 | N-Methyl-N-sec-butyltryptamine | |
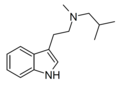 |
MiBT | artificial | H | CH3 | CH2CH(CH3)2 | N-Methyl-N-iso-butyltryptamine | |
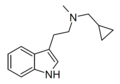 |
McPMT | artificial | H | CH3 | CH2C3H5 | N-Methyl-N-(cyclopropylmethyl)tryptamine | |
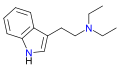 |
DET | artificial | H | CH2CH3 | CH2CH3 | N,N-diethyltryptamine | 61-51-8 |
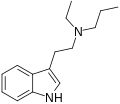 |
EPT | artificial | H | CH2CH3 | CH2CH2CH3 | N-Ethyl-N-propyltryptamine | 850032-68-7 |
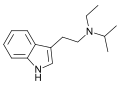 |
EiPT | artificial | H | CH2CH3 | CH(CH3)2 | N-Ethyl-N-isopropyltryptamine | 848130-11-0 |
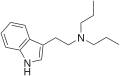 |
DPT | artificial | H | CH2CH2CH3 | CH2CH2CH3 | N,N-dipropyltryptamine | 61-52-9 |
 |
PiPT | artificial | H | CH2CH2CH3 | CH(CH3)2 | N-Propyl-N-isopropyltryptamine | 1354632-00-0 |
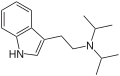 |
DiPT | artificial | H | CH(CH3)2 | CH(CH3)2 | N,N-diisopropyltryptamine | 14780-24-6 |
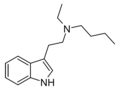 |
EBT | artificial | H | CH2CH3 | (CH2)3CH3 | N-ethyl-N-butyltryptamine | |
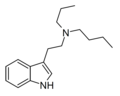 |
PBT | artificial | H | CH2CH2CH3 | (CH2)3CH3 | N-propyl-N-butyltryptamine | |
 |
iPsBT | artificial | H | CH(CH3)2 | CH(CH3)CH2CH3 | N-isopropyl-N-sec-butyltryptamine | |
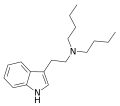 |
DBT | artificial | H | (CH2)3CH3 | (CH2)3CH3 | N,N-dibutyltryptamine | 15741-77-2 |
 |
DIBT | artificial | H | CH2CH(CH3)2 | CH2CH(CH3)2 | N,N-diisobutyltryptamine | 63938-64-7 |
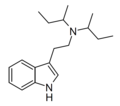 |
DSBT | artificial | H | CH(CH3)CH2CH3 | CH(CH3)CH2CH3 | N,N-disecbutyltryptamine | |
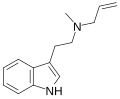 |
MALT | artificial | H | CH3 | H2C=CH-CH2 | N-methyl-N-allyltryptamine | |
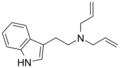 |
DALT | artificial | H | H2C=CH-CH2 | H2C=CH-CH2 | N,N-diallyltryptamine | 60676-77-9 |
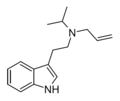 |
ALiPT | artificial | H | H2C=CH-CH2 | CH(CH3)2 | N-allyl-N-isopropyltryptamine | |
 |
2-Methyl-DMT | artificial | 2-CH3 | CH3 | CH3 | (2-(2-methyl-1H-indol-3-yl)-1-methyl-ethyl)dimethylamine | 1080-95-1 |
 |
2-Me-DET | artificial | 2-CH3 | CH2CH3 | CH2CH3 | N,N-Diethyl-2-(2-methyl-1H-indol-3-yl)ethan-1-amine | 26628-88-6 |
 |
4-Amino-DMT | artificial | 4-NH2 | CH3 | CH3 | 4-amino-N,N-dimethyltryptamine | 60331-61-5 |
 |
4-Methyl-DMT | artificial | 4-CH3 | CH3 | CH3 | 4,N,N-trimethyltryptamine | 28289-23-8 |
 |
4-MeO-DMT | artificial | 4-OCH3 | CH3 | CH3 | 4-methoxy-N,N-dimethyltryptamine | 3965-97-7 |
 |
4-MeO-MiPT | artificial | 4-OCH3 | CH3 | CH(CH3)2 | 4-methoxy-N-methyl-N-isopropyltryptamine | 96096-53-6 |
 |
4-MeO-DiPT | artificial | 4-OCH3 | CH(CH3)2 | CH(CH3)2 | 4-methoxy-N,N-diisopropyltryptamine | |
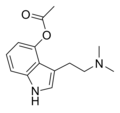 |
4-AcO-DMT | artificial | 4-OCOCH3 | CH3 | CH3 | 4-acetoxy-N,N-dimethyltryptamine | 92292-84-7 |
 |
4-PrO-DMT | artificial | 4-OCOCH2CH3 | CH3 | CH3 | 4-propionyloxy-N,N-dimethyltryptamine | 1373882-11-1 |
 |
4-HO-MET | artificial | 4-OH | CH3 | CH2CH3 | 4-hydroxy-N-methyl-N-ethyltryptamine | 77872-41-4 |
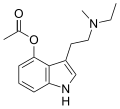 |
4-Acetoxy-MET | artificial | 4-OCOCH3 | CH3 | CH2CH3 | 4-acetoxy-N-methyl-N-ethyltryptamine | 1445751-40-5 |
 |
4-PO-MET | artificial | 4-OPO3H2 | CH3 | CH2CH3 | 4-phosphoryloxy-N-methyl-N-ethyltryptamine | |
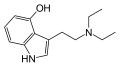 |
4-HO-DET | artificial | 4-OH | CH2CH3 | CH2CH3 | 4-hydroxy-N,N-diethyltryptamine | 22204-89-3 |
 |
4-Acetoxy-DET | artificial | 4-OCOCH3 | CH2CH3 | CH2CH3 | 4-acetoxy-N,N-diethyltryptamine | 1135424-15-5 |
 |
4-PO-DET | artificial | 4-OPO3H2 | CH2CH3 | CH2CH3 | 4-phosphoryloxy-N,N-diethyltryptamine | 60480-02-6 |
 |
4-HO-EPT | artificial | 4-OH | CH2CH3 | CH2CH2CH3 | 4-hydroxy-N-ethyl-N-propyltryptamine | 2595431-59-5 |
 |
4-PO-EPT | artificial | 4-OPO3H2 | CH2CH3 | CH2CH2CH3 | 4-phosphoryloxy-N-ethyl-N-propyltryptamine | |
 |
4-AcO-EiPT | artificial | 4-OCOCH3 | CH2CH3 | CH(CH3)2 | 4-acetoxy-N-ethyl-N-isopropyltryptamine | |
 |
4-HO-MPT | artificial | 4-OH | CH3 | CH2CH2CH3 | 4-hydroxy-N-methyl-N-propyltryptamine | 763035-03-6 |
 |
4-HO-MiPT | artificial | 4-OH | CH(CH3)2 | CH3 | 4-hydroxy-N-isopropyl-N-methyltryptamine | 77872-43-6 |
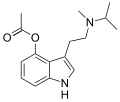 |
4-Acetoxy-MiPT | artificial | 4-OCOCH3 | CH3 | CH(CH3)2 | 4-acetoxy-N-methyl-N-isopropyltryptamine | 1024612-25-6 |
 |
4-HO-MALT [2] | artificial | 4-OH | CH3 | H2C=CH-CH2 | 4-hydroxy-N-Methyl-N-allyltryptamine | |
 |
4-AcO-MALT [3] | artificial | 4-OCOCH3 | CH3 | H2C=CH-CH2 | 4-acetoxy-N-Methyl-N-allyltryptamine | |
 |
4-HO-MSBT | artificial | 4-OH | CH(CH3)CH2CH3 | CH3 | 4-hydroxy-N-sec-butyl-N-methyltryptamine | |
 |
4-HO-McPT | artificial | 4-OH | C3H5 | CH3 | 4-hydroxy-N-cyclopropyl-N-methyltryptamine | |
 |
4-HO-McPeT | artificial | 4-OH | C5H9 | CH3 | 4-hydroxy-N-cyclopentyl-N-methyltryptamine | 77872-48-1 |
 |
4-HO-McPMT [4] | artificial | 4-OH | CH2C3H5 | CH3 | 4-hydroxy-N-cyclopropylmethyl-N-methyltryptamine | |
 |
4-HO-DPT | artificial | 4-OH | CH2CH2CH3 | CH2CH2CH3 | 4-hydroxy-N,N-dipropyltryptamine | 63065-88-3 |
 |
4-AcO-DPT | artificial | 4-OCOCH3 | CH2CH2CH3 | CH2CH2CH3 | 4-acetoxy-N,N-dipropyltryptamine | 1445751-75-6 |
 |
4-HO-PiPT | artificial | 4-OH | CH2CH2CH3 | CH(CH3)2 | 4-hydroxy-N-propyl-N-isopropyltryptamine | |
 |
4-HO-DIPT | artificial | 4-OH | CH(CH3)2 | CH(CH3)2 | 4-hydroxy-N,N-diisopropyltryptamine | 132328-45-1 |
 |
4-Acetoxy-DiPT | artificial | 4-OCOCH3 | CH(CH3)2 | CH(CH3)2 | 4-acetoxy-N,N-diisopropyltryptamine | 936015-60-0 |
 |
4-PrO-DiPT | artificial | 4-OCOCH2CH3 | CH(CH3)2 | CH(CH3)2 | 4-propionyloxy-N,N-diisopropyltryptamine | 1373882-13-3 |
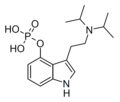 |
4-PO-DiPT | artificial | 4-OPO3H2 | CH(CH3)2 | CH(CH3)2 | 4-phosphoryloxy-N,N-diisopropyltryptamine | 1373882-09-7 |
 |
4-HO-DALT | artificial | 4-OH | H2C=CH-CH2 | H2C=CH-CH2 | 4-hydroxy-N,N-diallyltryptamine | |
 |
4-AcO-DALT | artificial | 4-OCOCH3 | H2C=CH-CH2 | H2C=CH-CH2 | 4-acetoxy-N,N-diallyltryptamine | 1445751-71-2 |
 |
4-HO-DBT | artificial | 4-OH | (CH2)3CH3 | (CH2)3CH3 | 4-hydroxy-N,N-dibutyltryptamine | 63065-89-4 |
 |
4-HO-DIBT | artificial | 4-OH | CH2CH(CH3)2 | CH2CH(CH3)2 | 4-hydroxy-N,N-diisobutyltryptamine | |
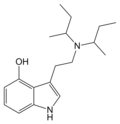 |
4-HO-DSBT | artificial | 4-OH | CH(CH3)CH2CH3 | CH(CH3)CH2CH3 | 4-hydroxy-N,N-disecbutyltryptamine | 127507-01-1 |
 |
5-MeO-MET | artificial | 5-OCH3 | CH2CH3 | CH3 | 5-methoxy-N-Methyl-N-ethyltryptamine | 16977-53-0 |
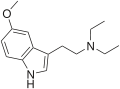 |
5-MeO-DET | artificial | 5-OCH3 | CH2CH3 | CH2CH3 | 5-methoxy-N,N-diethyltryptamine | 2454-70-8 |
 |
5-MeO-MPT | artificial | 5-OCH3 | CH3 | CH2CH2CH3 | 5-methoxy-N-methyl-N-propyltryptamine | |
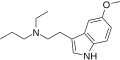 |
5-MeO-EPT | artificial | 5-OCH3 | CH2CH3 | CH2CH2CH3 | 5-methoxy-N-ethyl-N-propyltryptamine | 850032-67-6 |
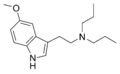 |
5-MeO-DPT | artificial | 5-OCH3 | CH2CH2CH3 | CH2CH2CH3 | 5-methoxy-N,N-dipropyltryptamine | 69496-75-9 |
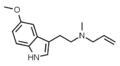 |
5-MeO-MALT | artificial | 5-OCH3 | H2C=CH-CH2 | CH3 | 5-methoxy-N-Methyl-N-allyltryptamine | 1373918-64-9 |
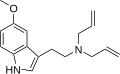 |
5-MeO-DALT | artificial | 5-OCH3 | H2C=CH-CH2 | H2C=CH-CH2 | 5-methoxy-N,N-diallyltryptamine | 928822-98-4 |
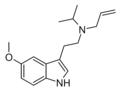 |
5-MeO-ALiPT | artificial | 5-OCH3 | H2C=CH-CH2 | CH2CH(CH3)2 | 5-methoxy-N-allyl-N-isopropyltryptamine | |
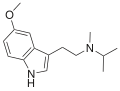 |
5-MeO-MiPT | artificial | 5-OCH3 | CH3 | CH(CH3)2 | 5-methoxy-N,N-methylisopropyltryptamine | 96096-55-8 |
 |
5,6-MeO-MiPT | artificial | 5-OCH3, 6-OCH3 | CH3 | CH(CH3)2 | 5,6-dimethoxy-N,N-methylisopropyltryptamine | |
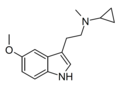 |
5-MeO-McPT | artificial | 5-OCH3 | CH3 | C3H5 | 5-methoxy-N-methyl-N-cyclopropyltryptamine | |
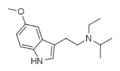 |
5-MeO-EiPT | artificial | 5-OCH3 | CH2CH3 | CH(CH3)2 | 5-methoxy-N-ethyl-N-isopropyltryptamine | 850032-66-5 |
 |
5-MeO-PiPT | artificial | 5-OCH3 | CH2CH2CH3 | CH(CH3)2 | 5-methoxy-N-propyl-N-isopropyltryptamine | |
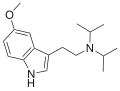 |
5-MeO-DIPT | artificial | 5-OCH3 | CH(CH3)2 | CH(CH3)2 | 5-methoxy-N,N-diisopropyltryptamine | 4021-34-5 |
 |
5-MeO-DBT | artificial | 5-OCH3 | (CH2)3CH3 | (CH2)3CH3 | 5-methoxy-N,N-dibutyltryptamine | 73785-42-9 |
 |
5-MeS-DMT | artificial | 5-SCH3 | CH3 | CH3 | 5-methylthio-N,N-dimethyltryptamine | 5102-11-4 |
 |
5-AcO-DMT | artificial | 5-OCOCH3 | CH3 | CH3 | 5-acetoxy-N,N-dimethyltryptamine | 16977-50-7 |
 |
5-AcO-MET [5] | artificial | 5-OCOCH3 | CH3 | CH2CH3 | 5-acetoxy-N-methyl-N-ethyltryptamine | |
 |
5-AcO-DET | artificial | 5-OCOCH3 | CH2CH3 | CH2CH3 | 5-acetoxy-N,N-diethyltryptamine | |
 |
5-AcO-EPT [6] | artificial | 5-OCOCH3 | CH2CH3 | CH2CH2CH3 | 5-acetoxy-N-ethyl-N-propyltryptamine | |
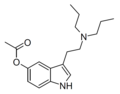 |
5-AcO-DPT | artificial | 5-OCOCH3 | CH2CH2CH3 | CH2CH2CH3 | 5-acetoxy-N,N-dipropyltryptamine | |
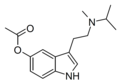 |
5-AcO-MiPT | artificial | 5-OCOCH3 | CH3 | CH(CH3)2 | 5-acetoxy-N-methyl-N-isopropyltryptamine | |
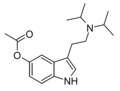 |
5-AcO-DiPT | artificial | 5-OCOCH3 | CH(CH3)2 | CH(CH3)2 | 5-acetoxy-N,N-diisopropyltryptamine | |
 |
5-Ethoxy-DMT | artificial | 5-OCH2CH3 | CH3 | CH3 | 5-ethoxy-N,N-dimethyltryptamine | 855245-09-9 |
 |
5-Ethoxy-MET | artificial | 5-OCH2CH3 | CH3 | CH2CH3 | 5-ethoxy-N-methyl-N-ethyltryptamine | |
 |
5-Ethoxy-DET | artificial | 5-OCH2CH3 | CH2CH3 | CH2CH3 | 5-ethoxy-N,N-diethyltryptamine | |
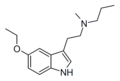 |
5-Ethoxy-MPT | artificial | 5-OCH2CH3 | CH3 | CH2CH2CH3 | 5-ethoxy-N-methyl-N-propyltryptamine | |
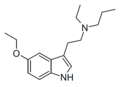 |
5-Ethoxy-EPT | artificial | 5-OCH2CH3 | CH2CH3 | CH2CH2CH3 | 5-ethoxy-N-ethyl-N-propyltryptamine | |
 |
5-Ethoxy-DPT | artificial | 5-OCH2CH3 | CH2CH2CH3 | CH2CH3 | 5-ethoxy-N,N-dipropyltryptamine | |
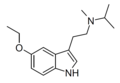 |
5-Ethoxy-MiPT | artificial | 5-OCH2CH3 | CH3 | CH(CH3)2 | 5-ethoxy-N-methyl-N-isopropyltryptamine | |
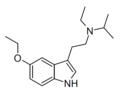 |
5-Ethoxy-EiPT | artificial | 5-OCH2CH3 | CH2CH3 | CH(CH3)2 | 5-ethoxy-N-ethyl-N-isopropyltryptamine | |
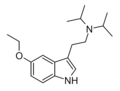 |
5-Ethoxy-DiPT | artificial | 5-OCH2CH3 | CH(CH3)2 | CH(CH3)2 | 5-ethoxy-N,N-diisopropyltryptamine | |
 |
5-Ethoxy-DALT | artificial | 5-OCH2CH3 | H2C=CH-CH2 | H2C=CH-CH2 | 5-ethoxy-N,N-diallyltryptamine | |
 |
5-BnO-DMT | artificial | 5-OCH2C6H5 | CH3 | CH3 | 5-benzyloxy-N,N-dimethyltryptamine | 101832-88-6 |
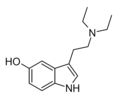 |
5-HO-DET | artificial | 5-OH | CH2CH3 | CH2CH3 | 5-hydroxy-N,N-diethyltryptamine | 14009-42-8 |
 |
5-HO-DPT | artificial | 5-OH | CH2CH2CH3 | CH2CH2CH3 | 5-hydroxy-N,N-dipropyltryptamine | 36288-75-2 |
 |
5-HO-MiPT | artificial | 5-OH | CH3 | CH(CH3)2 | 5-hydroxy-N-methyl-N-isopropyltryptamine | |
 |
5-HO-DiPT | artificial | 5-OH | CH(CH3)2 | CH(CH3)2 | 5-hydroxy-N,N-diisopropyltryptamine | 36288-76-3 |
 |
5-Methyl-DMT (5,N,N-TMT) | artificial | 5-CH3 | CH3 | CH3 | 5,N,N-trimethyltryptamine | 22120-39-4 |
 |
5-Ethyl-DMT | artificial | 5-CH2CH3 | CH3 | CH3 | 5-ethyl-N,N-dimethyltryptamine | 171783-25-8 |
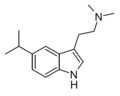 |
5-Isopropyl-DMT | artificial | 5-CH(CH3)2 | CH3 | CH3 | 5-isopropyl-N,N-dimethyltryptamine | 156281-04-8 |
 |
5-(t-Butyl)-DMT [7] | artificial | 5-C(CH3)3 | CH3 | CH3 | 5-(tert-butyl)-N,N-dimethyltryptamine | |
 |
5-Fluoro-DMT | artificial | 5-F | CH3 | CH3 | 5-fluoro-N,N-dimethyltryptamine | 22120-36-1 |
 |
5-Fluoro-MET | artificial | 5-F | CH3 | CH2CH3 | 5-fluoro-N-methyl-N-ethyltryptamine | |
 |
5-Fluoro-DET | artificial | 5-F | CH2CH3 | CH2CH3 | 5-fluoro-N,N-diethyltryptamine | |
 |
5-Fluoro-EPT | artificial | 5-F | CH2CH3 | CH2CH2CH3 | 5-fluoro-N-ethyl-N-propyltryptamine | |
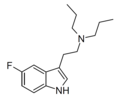 |
5-Fluoro-DPT | artificial | 5-F | CH2CH2CH3 | CH2CH2CH3 | 5-fluoro-N,N-dipropyltryptamine | |
 |
5-Fluoro-DiPT | artificial | 5-F | CH(CH3)2 | CH(CH3)2 | 5-fluoro-N,N-diisoproptryptamine | |
 |
5-Chloro-DMT | artificial | 5-Cl | CH3 | CH3 | 5-chloro-N,N-dimethyltryptamine | 22120-32-7 |
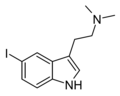 |
5-Iodo-DMT | artificial | 5-I | CH3 | CH3 | 5-iodo-N,N-dimethyltryptamine | 22120-38-3 |
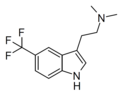 |
5-TFM-DMT | artificial | 5-CF3 | CH3 | CH3 | 5-(trifluoromethyl)-N,N-dimethyltryptamine | 2418713-32-1 |
 |
5-Nitro-DMT | artificial | 5-NO2 | CH3 | CH3 | 5-nitro-N,N-dimethyltryptamine | 69937-13-9 |
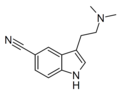 |
5-Cyano-DMT | artificial | 5-C≡N | CH3 | CH3 | 5-cyano-N,N-dimethyltryptamine | 17380-42-6 |
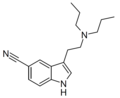 |
5-Cyano-DPT | artificial | 5-C≡N | CH2CH2CH3 | CH2CH2CH3 | 5-cyano-N,N-dipropyltryptamine | 74885-19-1 |
 |
Almotriptan | artificial | 5-(CH2SO2N(CH2)4) | CH3 | CH3 | N,N-dimethyl-2- [5-(pyrrolidin-1-ylsulfonylmethyl)- 1H-indol-3-yl]-ethanamine | 154323-57-6 |
 |
Rizatriptan | artificial | 5-(CH2(N3(CH)2)) | CH3 | CH3 | N,N-dimethyl-2-[5-(1H-1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethanamine | 145202-66-0 |
 |
Sumatriptan | artificial | 5-(CH2SO2NHCH3) | CH3 | CH3 | 1-[3-(2-Dimethylaminoethyl)-1H-indol-5-yl]-N-methyl-methanesulfonamide | 103628-46-2 |
 |
Zolmitriptan | artificial | 5-(CHNHC=OOCH2) | CH3 | CH3 | 5-( 4-(S)-1,3-oxazolidin-2-one)-N,N-dimethyltryptamine | 139264-17-8 |
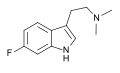 |
6-Fluoro-DMT | artificial | 6-F | CH3 | CH3 | 6-fluoro-N,N-dimethyltryptamine | 1511-31-5 |
 |
6-Fluoro-DET[8] | artificial | 6-F | CH2CH3 | CH2CH3 | 6-fluoro-N,N-diethyltryptamine | 2836-69-3 |
 |
6-Chloro-DMT | artificial | 6-Cl | CH3 | CH3 | 6-chloro-N,N-dimethyltryptamine | 25390-72-1 |
 |
6-Methyl-DMT | artificial | 6-CH3 | CH3 | CH3 | 6,N,N-trimethyltryptamine | |
 |
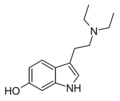
6-Hydroxy-DMT | artificial | 6-OH | CH3 | CH3 | 6-hydroxy-N,N-dimethyltryptamine | 1476-33-1 |
 |
6-Hydroxy-DET | artificial | 6-OH | CH3 | CH3 | 6-hydroxy-N,N-diethyltryptamine | 1476-59-1 |
 |
6-Methoxy-DMT | artificial | 6-OCH3 | CH3 | CH3 | 6-methoxy-N,N-dimethyltryptamine | 2426-88-2 |
 |
7-Methyl-DMT | artificial | 7-CH3 | CH3 | CH3 | 7,N,N-trimethyltryptamine | 65882-39-5 |
 |
7-Ethyl-DMT | artificial | 7-CH2CH3 | CH3 | CH3 | 7-ethyl-N,N-dimethyltryptamine | |
 |
7-Chloro-DMT | artificial | 7-Cl | CH3 | CH3 | 7-chloro-N,N-dimethyltryptamine | |
 |
7-Bromo-DMT[9] | artificial | 7-Br | CH3 | CH3 | 7-bromo-N,N-dimethyltryptamine | 74798-68-8 |
 |
7-Methoxy-DMT | artificial | 7-OCH3 | CH3 | CH3 | 7-methoxy-N,N-dimethyltryptamine | |
 |
7-Methoxy-MiPT | artificial | 7-OCH3 | CH3 | CH(CH3)2 | 7-methoxy-N-methyl-N-isopropyltryptamine | |
 |
1-Methylpsilocin | artificial | 1-CH3, 4-OH | CH3 | CH3 | 1-Methyl-3-[2-(N,N-dimethylamino)ethyl]-4-hydroxyindole | 1465-16-3 |
 |
1-Methyl-5-MeO-DiPT | artificial | 1-CH3, 5-OCH3 | CH(CH3)2 | CH(CH3)2 | 1-methyl-5-methoxy-N,N-diisopropyltryptamine | 1373882-10-0 |
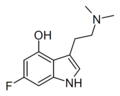 |
6-Fluoropsilocin | artificial | 4-OH,6-F | CH3 | CH3 | 4-hydroxy-6-fluoro-N,N-dimethyltryptamine | 312314-12-8 |
 |
6-Fluoro-5-MeO-DMT | artificial | 5-OCH3,6-F | CH3 | CH3 | 5-methoxy-6-fluoro-N,N-dimethyltryptamine | |
 |
5-MeO-2-TMT | artificial | 2-CH3, 5-OCH3 | CH3 | CH3 | 2-(5-methoxy-2-methyl-H-indol-3-yl)-N,N-dimethylethanamine | 67292-68-6 |
 |
5-Methoxy-7,N,N-trimethyltryptamine | artificial | 5-OCH3, 7-CH3 | CH3 | CH3 | 5-Methoxy-7,N,N-trimethyltryptamine | 61018-77-7 |
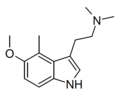 |
5-Methoxy-4,N,N-trimethyltryptamine | artificial | 4-CH3, 5-OCH3 | CH3 | CH3 | 5-Methoxy-4,N,N-trimethyltryptamine | |
 |
4-HO-5-MeO-DMT | artificial | 4-OH, 5-OCH3 | CH3 | CH3 | 4-Hydroxy-5-methoxy-N,N-dimethyltryptamine | 2433-31-0 |
 |
4-F-5-MeO-DMT | artificial | 4-F, 5-OCH3 | CH3 | CH3 | 4-Fluoro-5-Methoxy-N,N-dimethyltryptamine | 312314-18-4 |
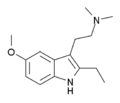 |
EMDT | artificial | 2-CH2CH3, 5-OCH3 | CH3 | CH3 | 2-(2-ethyl-5-methoxy-1H-indol-3-yl)-N,N-dimethylethanamine | 263744-72-5 |
 |
ST-1936 | artificial | 2-CH3, 5-Cl | CH3 | CH3 | 2-(2-methyl-5-chloro-1H-indol-3-yl)-N,N-dimethylethanamine | 1210-81-7 |
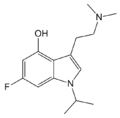 |
O-4310 | artificial | 1-CH(CH3)2, 4-OH, 6-F | CH3 | CH3 | 3-[2-(dimethylamino)ethyl]-6-fluoro-1-isopropyl-1H-indol-4-ol | 885671-63-6 |
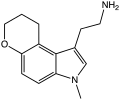 |
CP-132,484 | artificial | 1-methyl-4,5-(OCH2CH2CH2) | H | H | 1-(2-aminoethyl)-3-methyl-8,9-dihydropyrano(3,2-e)indole | 143508-76-3 |
 |
1-(2-Dimethylaminoethyl)dihydropyrano(3,2-e)indole | artificial | 4,5-(OCH2CH2CH2) | CH3 | CH3 | 1-(2-dimethylaminoethyl)-8,9-dihydropyrano[3,2-e]indole | 135360-97-3 |
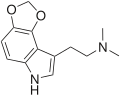 |
4,5-MDO-DMT | artificial | 4,5-(OCH2O) | CH3 | CH3 | 2-(2H,6H-[1,3]Dioxolo[4,5-e]indol-8-yl)-N,N-dimethylethan-1-amine | 81249-30-1 |
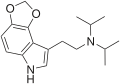 |
4,5-MDO-DiPT | artificial | 4,5-(OCH2O) | CH(CH3)2 | CH(CH3)2 | N-[2-(2H,6H-[1,3]Dioxolo[4,5-e]indol-8-yl)ethyl]-N-(propan-2-yl)propan-2-amine | |
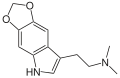 |
5,6-MDO-DMT | artificial | 5,6-(OCH2O) | CH3 | CH3 | 2-(2H,5H-[1,3]Dioxolo[4,5-f]indol-7-yl)-N,N-dimethylethan-1-amine | |
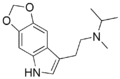 |
5,6-MDO-MiPT | artificial | 5,6-(OCH2O) | CH3 | CH(CH3)2 | N-[2-(2H,5H-[1,3]Dioxolo[4,5-f]indol-7-yl)ethyl]-N-methylpropan-2-amine | |
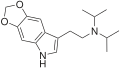 |
5,6-MDO-DiPT | artificial | 5,6-(OCH2O) | CH(CH3)2 | CH(CH3)2 | N-[2-(2H,5H-[1,3]Dioxolo[4,5-f]indol-7-yl)ethyl]-N-(propan-2-yl)propan-2-amine | |
| Chemical Structure | Short Name | Origin | Ring Substitution | RN1 | RN2 | Full Name | CAS Number |
List of substituted α-alkyltryptamines[]
α-Alkyltryptamines are a group of substituted tryptamines which possess an alkyl group, such as a methyl or ethyl group, attached at the alpha carbon, and in most cases no substitution on the amine nitrogen.[10][11][12] α-Alkylation of tryptamine makes it much more metabolically stable and resistant to degradation by monoamine oxidase, resulting in increased potency and greatly lengthened half-life.[12] This is analogous to α-methylation of phenethylamine into amphetamine.[12]
Many α-alkyltryptamines are drugs, acting as monoamine releasing agents, non-selective serotonin receptor agonists, and/or monoamine oxidase inhibitors,[13][14][15][16] and produce psychostimulant, entactogen, and/or psychedelic effects.[10][11][12] The most well-known of these agents are α-methyltryptamine (αMT) and α-ethyltryptamine (αET), both of which were used clinically as antidepressants for a brief period of time in the past and are abused as recreational drugs.[11][12] In accordance with its action as a dual releasing agent of serotonin and dopamine, αET has been found to produce serotonergic neurotoxicity similarly to amphetamines like MDMA and PCA, and the same is also likely to hold true for other serotonin and dopamine-releasing α-alkyltryptamines such as αMT, 5-MeO-αMT, and various others.[17]
| Structure | Common name | Chemical name | CAS number |
|---|---|---|---|

|
Tryptophan | (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid | 73-22-3 |

|
5-Hydroxytryptophan | 2-amino-3-(5-hydroxy-1H-indol-3-yl)propanoic acid | 4350-09-8 |

|
αMT | 1-(1H-Indol-3-yl)propan-2-amine | 299-26-3 |

|
4-HO-αMT | 3-(2-aminopropyl)-1H-indol-4-ol | 15066-09-8 |

|
4-Methyl-αMT | 1-methyl-2-(4-methyl-1H-indol-3-yl)-ethylamine | 3569-29-7 |

|
5-Fluoro-αMT | 1-(5-fluoro-1H-indol-3-yl)propan-2-amine | 712-08-3 |
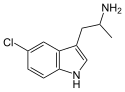
|
5-Chloro-αMT | 1-(5-Chloro-1H-indol-3-yl)propan-2-amine | 712-07-2 |
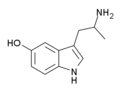
|
5-HO-αMT (αMS/α-methyl-5-HT) | 3-(2-aminopropyl)-1H-indol-5-ol | 304-52-9 |
|
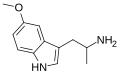
5-MeO-αMT | 1-(5-methoxy-1H-indol-3-yl)propan-2-amine | 1137-04-8 |

|
5-Ethoxy-αMT | 1-(5-ethoxy-1H-indol-3-yl)propan-2-amine | 101832-83-1 |

|
5-Isopropoxy-αMT | 1-{5-[(propan-2-yl)oxy]-1H-indol-3-yl}propan-2-amine | |

|
BW-723C86 | 1-[5-(2-Thienylmethoxy)-1H-indol-3-yl]-2-propanamine | 160521-72-2 |

|
6-Fluoro-αMT | 1-(6-fluoro-1H-indol-3-yl)propan-2-amine | 712-11-8 |

|
7-Chloro-AMT | 1-(7-chloro-1H-indol-3-yl)propan-2-amine | 711-99-9 |

|
AL-37350A (4,5-dihydropyrano-αMT) | (S)-(+)-1-(2-Aminopropyl)-8,9-dihydropyrano[3,2-e]indole | 362603-40-5 |

|
Compound 5 [18] | 1-(3H-benzo[e]indol-1-yl)propan-2-amine | |
|
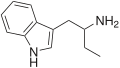
αET | 1-(1H-indol-3-yl)butan-2-amine | 2235-90-7 |
|
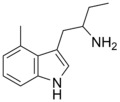
4-Methyl-αET | 1-(4-Methyl-1H-indol-3-yl)butan-2-amine | 28289-30-7 |

|
4-HO-αET | 1-(4-hydroxy-1H-indol-3-yl)butan-2-amine | 28289-28-3 |
|
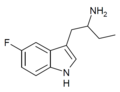
5-Fluoro-αET | 1-(5-fluoro-1H-indol-3-yl)butan-2-amine | 1380137-98-3 |

|
1-(5-methyl-1H-indol-3-yl)butan-2-amine | 1380148-21-9 | |

|
5-MeO-αET | 1-(5-methoxy-1H-indol-3-yl)butan-2-amine | 4765-10-0 |

|
7-Methyl-αET | 1-(7-methyl-1H-indol-3-yl)butan-2-amine | 13712-80-6 |

|
N-Methyl-5-MeO-αMT (α,N,O-TMS/α,N,O-trimethyl-5-HT) | [1-(5-methoxy-1H-indol-3-yl)propan-2-yl](methyl)amine | 4822-13-3 |
|
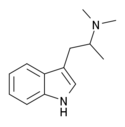
N,N-Dimethyl-αMT (α,N,N-TMT) | (2-(1H-Indol-3-yl)-1-methyl-ethyl)dimethylamine | |
|
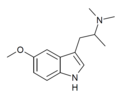
(5-MeO-α,N,N-TMT) | (2-(5-methoxy-1H-Indol-3-yl)-1-methyl-ethyl)dimethylamine | 101831-90-7 |

|
αMDiPT | (2-(1H-Indol-3-yl)-1-methyl-ethyl)diisopropylamine | |

|
MPMI[19] | 3-[(1-methylpyrrolidin-2-yl)methyl]-1H-indole | 143321-54-4 |

|
Lucigenol | (R)-3-(N-methylpyrrolidin-2-ylmethyl)-4-hydoxyindole | 250672-65-2 |

|
5-MeO-MPMI | 5-Methoxy-3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-1H-indole | 143321-57-7 |

|
5-F-MPMI | 5-fluoro-3-[(1-methylpyrrolidin-2-yl)methyl]-1H-indole | |

|
5-Br-MPMI | 5-bromo-3-[(1-methylpyrrolidin-2-yl)methyl]-1H-indole | 143322-57-0 |

|
Eletriptan | 3-{[(2R)-1-methylpyrrolidin-2-yl]methyl}-5-[2-(benzenesulfonyl)ethyl]-1H-indole | 143322-58-1 |
Related compounds[]
A number of related compounds are known, with a similar structure but having the indole core flipped and/or replaced with related cores such as indoline, indazole or benzofuran. These similarly are primarily active as agonists at the 5-HT2 family of serotonin receptors, with applications in the treatment of glaucoma, cluster headaches or as anorectics.
| Structure | Common name | Chemical name | CAS number |
|---|---|---|---|

|
Dimemebfe | 2-(5-Methoxy-1-benzofuran-3-yl)-N,N-dimethylethanamine | 140853-58-3 |
|
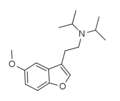
5-MeO-DiBF | N-[2-(5-Methoxy-1-benzofuran-3-yl)ethyl]-N-(propan-2-yl)propan-2-amine | |

|
3-APB | 3-(2-aminopropyl)benzofuran | 105909-13-5 |
|
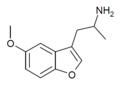
Mebfap | 3-(2-aminopropyl)-5-methoxybenzofuran | 140853-59-4 |

|
Ro60-0175 | (S)-(6-chloro-5-fluoro-1H-indol-1-yl)propan-2-amine | 169675-09-6 |
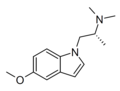
|
AAZ-A-154 | (2R)-1-(5-methoxy-1H-indol-1-yl)-N,N-dimethylpropan-2-amine | |
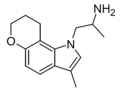
|
Example 1 [20] | 1-(3-methyl-8,9-dihydropyrano[2,3-g]indol-1(7H)-yl)propan-2-amine | |
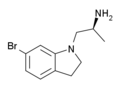
|
VER-3323 | (2S)-1-(6-bromo-2,3-dihydroindol-1-yl)propan-2-amine | 259857-99-3 |

|
AL-34662 | 1-((S)-2-Aminopropyl)-1H-indazol-6-ol | 210580-75-9 |

|
O-methyl-AL-34662 | 1-((S)-6-methoxy-2-aminopropyl)-1H-indazole | 210580-60-2 |

|
7-methyl-AL-34662 | 1-((S)-2-Aminopropyl)-7-methyl-1H-indazol-6-ol | 874668-67-4 |
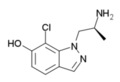
|
7-chloro-AL-34662 | 1-((S)-2-Aminopropyl)-7-chloro-1H-indazol-6-ol | 874881-86-4 |
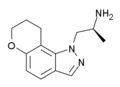
|
AL-38022A | (S)-2-(8,9-dihydro-7H-pyrano[2,3-g]indazol-1-yl)-1-methylethylamine | 478132-11-5 |

|
Example 9 [21] | (S)-α-methyl-pyrano[2,3-g]indazole-1(7H)-ethanamine | 478132-12-6 |
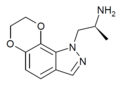
|
Example 3 [22] | (S)-7,8-dihydro-α-methyl-1H-[1,4]dioxino[2,3-g]indazole-1-ethanamine | 890087-75-9 |

|
Example 1 [23] | (S)-8,9-dihydro-α,9-dimethylpyrazolo[3,4-f][1,4]benzoxazine-1(7H)-ethanamine | 1373917-69-1 |

|
YM-348 | (2S)-1-(7-ethyl-1H-furo[2,3-g]indazol-1-yl)propan-2-amine | 372163-84-3 |
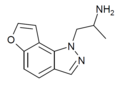
|
2-desethyl-YM-348 [24] | (2S)-1-(1H-furo[2,3-g]indazol-1-yl)propan-2-amine | 748116-94-1 |
Further reading[]
- TiHKAL
See also[]
- Ergoline
- Lysergamide
- Iboga alkaloid
- Substituted amphetamine
- Substituted benzofuran
- Substituted cathinone
- Substituted methylenedioxyphenethylamine
- Substituted phenethylamine
- 2C, DOx, 25-NB
References[]
- ^ Toro-Sazo M, Brea J, Loza MI, Cimadevila M, Cassels BK (2019). "5-HT2 receptor binding, functional activity and selectivity in N-benzyltryptamines". PLOS ONE. 14 (1): e0209804. doi:10.1371/journal.pone.0209804. PMC 6328172. PMID 30629611.
- ^ Klein AK, et al. Investigation of the Structure–Activity Relationships of Psilocybin Analogues. ACS Pharmacol. Transl. Sci. 2021; 4(2): 533–542. doi:10.1021/acsptsci.0c00176
- ^ Pham DNK, et al. Psilacetin derivatives: fumarate salts of the methyl–ethyl, methyl–allyl and diallyl variants of the psilocin prodrug. Acta Cryst. (2021). E77, 101-106. doi:10.1107/S2056989021000116
- ^ KOZIKOWSKI, Alan. SHAPIRO, Gideon. TUECKMANTEL, Werner. MCCORVY, John. 3-(2-(AMINOETHYL)-INDOL-4-OL DERIVATIVES, METHODS OF PREPARATION THEREOF, AND THE USE AS 5-HT2 RECEPTOR MODULATORS. Patent WO 2021/179091
- ^ Stamets PE. Tryptamine Compositions for Enhancing Neurite Outgrowth. WO 2021/101926
- ^ Kruegel AC, Sporn J. Specific Tryptamines for use in the Treatment of Mood Disorders. WO 2021/168082
- ^ Xu YC, Schaus JM, Walker C, Krushinski J, Adham N, Zgombick JM, Liang SX, Kohlman DT, Audia JE (February 1999). "N-Methyl-5-tert-butyltryptamine: A novel, highly potent 5-HT1D receptor agonist". Journal of Medicinal Chemistry. 42 (3): 526–31. doi:10.1021/jm9805945. PMID 9986723.
- ^ Rabin RA, Regina M, Doat M, Winter JC (May 2002). "5-HT2A receptor-stimulated phosphoinositide hydrolysis in the stimulus effects of hallucinogens". Pharmacology, Biochemistry, and Behavior. 72 (1–2): 29–37. doi:10.1016/s0091-3057(01)00720-1. PMID 11900766. S2CID 6480715.
- ^ Glennon RA, Schubert E, Jacyno JM, Rosecrans JA (November 1980). "Studies on several 7-substituted N,N-dimethyltryptamines". Journal of Medicinal Chemistry. 23 (11): 1222–6. doi:10.1021/jm00185a014. PMID 6779006.
- ^ a b Ries RK, Miller SC, Fiellin DA (2009). Principles of Addiction Medicine. Lippincott Williams & Wilkins. pp. 216–218. ISBN 978-0-7817-7477-2.
- ^ a b c Laing RR (2003). Hallucinogens: A Forensic Drug Handbook. Academic Press. pp. 102–. ISBN 978-0-12-433951-4.
- ^ a b c d e Lemke TL, Williams DA (24 January 2012). Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. pp. 641–. ISBN 978-1-60913-345-0.
- ^ Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology. 559 (2–3): 132–7. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ^ Blough BE, Landavazo A, Partilla JS, Decker AM, Page KM, Baumann MH, Rothman RB (October 2014). "Alpha-ethyltryptamines as dual dopamine-serotonin releasers". Bioorganic & Medicinal Chemistry Letters. 24 (19): 4754–4758. doi:10.1016/j.bmcl.2014.07.062. PMC 4211607. PMID 25193229.
- ^ Nonaka R, Nagai F, Ogata A, Satoh K (December 2007). "In vitro screening of psychoactive drugs by [(35)S]GTPgammaS binding in rat brain membranes". Biological & Pharmaceutical Bulletin. 30 (12): 2328–33. doi:10.1248/bpb.30.2328. PMID 18057721.
- ^ Feldman JM, Chapman B (December 1975). "Monoamine oxidase inhibitors: nature of their interaction with rabbit pancreatic islets to alter insluin secretion". Diabetologia. 11 (6): 487–94. doi:10.1007/bf01222097. PMID 1107123.
- ^ Huang XM, Johnson MP, Nichols DE (July 1991). "Reduction in brain serotonin markers by alpha-ethyltryptamine (Monase)". European Journal of Pharmacology. 200 (1): 187–90. doi:10.1016/0014-2999(91)90686-K. PMID 1722753.
- ^ Chang-Fong J, Addo J, Dukat M, Smith C, Mitchell NA, Herrick-Davis K, Teitler M, Glennon RA (January 2002). "Evaluation of isotryptamine derivatives at 5-HT(2) serotonin receptors". Bioorganic & Medicinal Chemistry Letters. 12 (2): 155–8. doi:10.1016/s0960-894x(01)00713-2. PMID 11755343.
- ^ Macor JE, Wythes MJ. Indole derivatives. US patent 5607951
- ^ US granted 7012090, Chen HH, May JA, "Pyranoindoles for treating glaucoma", published 17 March 2000, issued 14 March 2006, assigned to Alcon, Inc.
- ^ US granted 6881749, Chen HH, May JA, Severns BS, "Pyranoindazoles and their use for the treatment of glaucoma", published 3 June 2004, issued 19 April 2005, assigned to Alcon, Inc.
- ^ US granted 7425572, Chen HH, May JA, "Use of dioxindoindazoles and dioxoloindazoles for treating glaucoma", published 8 June 2006, issued 16 September 2008, assigned to Alcon, Inc.
- ^ US granted 7268131, Dantanarayana AP, May JA, "Substituted [1,4]oxazino[2,3-g]indazoles for the treatment of glaucoma", published 15 December 2005, issued 11 September 2007, assigned to Alcon, Inc.
- ^ Shimada I, Maeno K, Kazuta K, Kubota H, Kimizuka T, Kimura Y, et al. (February 2008). "Synthesis and structure-activity relationships of a series of substituted 2-(1H-furo[2,3-g]indazol-1-yl)ethylamine derivatives as 5-HT2C receptor agonists". Bioorganic & Medicinal Chemistry. 16 (4): 1966–82. doi:10.1016/j.bmc.2007.10.100. PMID 18035544.
- Tryptamines
- Functional groups