5-MeO-2-TMT Legal status
DE NpSG (Industrial and scientific use only) UK :Class A
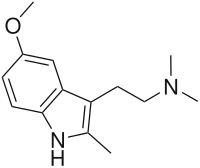
2-(5-methoxy-2-methyl-H-indol-3-yl)-N,N-dimethylethanamine
CAS Number PubChem CID ChemSpider UNII ChEMBL CompTox Dashboard (EPA ) Formula C 14 H 20 N 2 O Molar mass −1 3D model (JSmol )
CC1=C(C2=C(N1)C=CC(=C2)OC)CCN(C)C
InChI=1S/C14H20N2O/c1-10-12(7-8-16(2)3)13-9-11(17-4)5-6-14(13)15-10/h5-6,9,15H,7-8H2,1-4H3
Y Key:ACEHBQPPDDGCGZ-UHFFFAOYSA-N
Y
5-Methoxy-2,N ,N -trimethyltryptamine (5-MeO-2,N ,N -TMT , 5-MeO-TMT ) is a psychoactive drug of the tryptamine chemical class which acts as a psychedelic . It was first synthesized by Alexander Shulgin and reported in his book TiHKAL ("Tryptamines i Have Known And Loved").[1] compounds . This suggests that while the methyl group on the 2-position of the molecule has impaired the binding of metabolic enzymes like monoamine oxidase (MAO), it is also interfering with binding to and/or activation of the serotonin 5-HT2A receptor , the target responsible for mediating the hallucinogenic effects of such compounds.
See also [ ] 2,N ,N -Trimethyltryptamine (2,N ,N -TMT)5-MeO-7-TMT References [ ] External links [ ]
Tryptamines
1-Methylpsilocin 2,alpha-DMT 2-Me-DET 2-Methyl-5-HT 2,N,N-TMT 4,5-DHP-DMT 4,5-MDO-DMT 4,5-MDO-DiPT 4-AcO-DALT 4-AcO-DET 4-AcO-DMT 4-AcO-DiPT 4-AcO-MET 4-AcO-DPT 4-AcO-MiPT 4-F-5-MeO-DMT 4-HO-5-MeO-DMT 4-HO-DBT 4-HO-DET 4-HO-DiPT 4-HO-DPT 4-HO-DSBT 4-HO-EPT 4-HO-MET 4-HO-McPT 4-HO-McPeT 4-HO-MiPT 4-HO-MPMI 4-HO-MPT 4-HO-pyr-T 4-HO-αMT 4-Me-αET 4-Me-αMT 4-MeO-DMT 4-MeO-MiPT 4-PrO-DMT 5,6-MeO-MiPT 5,6-MDO-DiPT 5,6-MDO-DMT 5,6-MDO-MiPT 5,7-Dihydroxytryptamine 5-BT 5-Bromo-DMT 5-CT 5-Chloro-αMT 5-Chloro-DMT 5-Ethoxy-αMT 5-Ethoxy-DMT 5-Ethyl-DMT 5-Fluoro-AET 5-Fluoro-αMT 5-Fluoro-DET 5-Fluoro-DMT 5-Fluoro-EPT 5-Fluoro-MET 5-HO-αMT 5-HO-DiPT 5-HTP 5-MeS-DMT 5-Methoxytryptamine 5-MeO-7,N,N-TMT 5-MeO-2-TMT 5-MeO-αET 5-MeO-αMT 5-MeO-DALT 5-MeO-DBT 5-MeO-DET 5-MeO-DiPT 5-MeO-DMT 5-MeO-DPT 5-MeO-EiPT 5-MeO-EPT 5-MeO-MALT 5-MeO-MET 5-MeO-MiPT 5-MeO-MPMI 5-MeO-NMT 5-MeO-pyr-T 5-MeO-NBpBrT 5-Methyl-DMT 5-(Nonyloxy)tryptamine 6-Fluoro-αMT 6-Fluoro-DMT 6-Hydroxymelatonin 6-MeO-THH 7-Chloro-AMT 7-Methyl-α-ethyltryptamine 7-Methyl-DMT Acetryptine Aeruginascin αET Alpha,N-DMT α,N,N-Trimethyltryptamine Alpha,N,O-TMS AL-37350A αMT Baeocystin BNC-210 Bufotenidine Bufotenin (5-HO-DMT) BW-723C86 Convolutindole A CP-132,484 DALT DBT Desformylflustrabromine DET DiPT DPT E-6801 E-6837 Ethocybin EiPT EMDT EPT FGIN-127 FGIN-143 Harmaline HIOC Ibogaine Idalopirdine Indorenate Iprocin Lespedamine Luzindole MET Methylbutyltryptamine MiPT MPT Miprocin Melatonin MPMI MS-245 NAS N-Ethyltryptamine N-Feruloylserotonin NMT DMT Norbaeocystin Normelatonin N-t-Butyltryptamine O-4310 Oxypertine Plakohypaphorine PiPT Psilocin (4-HO-DMT) Psilocybin (4-PO-DMT) Pyr-T Rizatriptan RU-28306 Serotonin ST-1936 Sumatriptan Tryptamine Tryptophan Yohimbine Yuremamine Zolmitriptan
Hallucinogens
Psychedelics (5-HT2A
Benzofurans
2C-B-FLY 2CBFly-NBOMe 5-MeO-BFE 5-MeO-DiBF Bromo-DragonFLY F-2 F-22 TFMFly Lyserg‐ Phenethyl‐
Piperazines Tryptamines
alpha -alkyltryptaminesx -DALT
(Daltacetin) 4-AcO-DALT 5-MeO-DALT DALT x -DET
(Ethacetin) 4-AcO-DET (Ethocin) 4-HO-DET 5-MeO-DET (T-9) DET (Ethocybin) 4-PO-DET x -DiPT
(Ipracetin) 4-AcO-DiPT (Iprocin) 4-HO-DiPT 5-MeO-DiPT DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4-Propionyloxy-DMT 5-AcO-DMT 5-Bromo-DMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT Ibogaine-related
18-MAC 18-MC Coronaridine Ibogaine Ibogamine ME-18-MC Noribogaine Tabernanthine Voacangine x -MET
(Metocin) 4-HO-MET (Metocetin) 4-AcO-MET 5-MeO-MET MET x -MiPT
(Mipracetin) 4-AcO-MiPT (Miprocin) 4-HO-MiPT 5-Me-MiPT (Moxy) 5-MeO-MiPT MiPT Others
4-HO-DBT 4-HO-EPT 4-HO-McPT (Lucigenol) 4-HO-MPMI (Meprocin) 4-HO-MPT 5-MeO-EiPT 5-MeO-MALT 5-MeO-MPMI Aeruginascin Baeocystin DBT EiPT EPT MPT PiPT
Others
AL-38022A ALPHA Dimemebfe Efavirenz Glaucine Lorcaserin M-ALPHA RH-34 Also empathogens in general (e. g.: 5-APB , 5-MAPB , 6-APB and other substituted benzofurans ).
Dissociatives (NMDAR antagonists )
Arylcyclo‐
Ketamine-related PCP-related Others
Diarylethylamines Morphinans
Dextrallorphan Dextromethorphan Dextrorphan Racemethorphan Racemorphan Others
Deliriants (mAChR antagonists ) Others
Cannabinoids (CB1 agonists)
Natural
Salvinorin A THC (Dronabinol) THCV Synthetic
D2 agonists
Apomorphine Aporphine Bromocriptine Cabergoline Lisuride LSD Memantine Nuciferine Pergolide Phenethylamine Piribedil Pramipexole Ropinirole Rotigotine Salvinorin A Also indirect D2 agonists, such as dopamine reuptake inhibitors (cocaine , methylphenidate ), releasing agents (amphetamine , methamphetamine ), and precursors (levodopa ). GABAA
CI-966 Eszopiclone Ibotenic acid Muscimol (Amanita muscaria Zaleplon Zolpidem Zopiclone Inhalants (Mixed MOA )
Aliphatic hydrocarbons
Butane Gasoline Kerosene Propane Aromatic hydrocarbons
Ethers
Haloalkanes
Chlorofluorocarbons Chloroform κOR agonists
2-EMSB Alazocine Bremazocine Butorphan Butorphanol Cyclazocine Cyclorphan Cyprenorphine Diprenorphine Enadoline Herkinorin Heroin HZ-2 Ibogaine Ketazocine Levallorphan Levomethorphan Levorphanol LPK-26 Metazocine Morphine Nalbuphine Nalmefene Nalorphine Noribogaine Oxilorphan Pentazocine Phenazocine Proxorphan Racemethorphan Racemorphan Salvinorin A Spiradoline Tifluadom U-50488 U-69,593 Xorphanol Oneirogens
Calea zacatechichi Silene capensis Galantamine Others
Glaucine Isoaminile Noscapine Pukateine