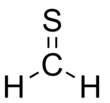
Thioformaldehyde
| |||
| Names | |||
|---|---|---|---|
| Other names
methanethial
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| CH2S | |||
| Molar mass | 46.09 | ||
| Appearance | unknown | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Thioformaldehyde is the organosulfur compound with the formula CH2S. This compound is very rarely observed because it oligomerizes to 1,3,5-trithiane, which is a stable colorless compound with the same empirical formula. Despite its instability under normal terrestrial conditions, the molecule has been observed in the interstellar medium[1] and has attracted much attention for its fundamental nature.[2] The tendency of thioformaldehyde to form chains and rings is a manifestation of the double bond rule.
Although thioformaldehyde tends to oligomerize, many metal complexes are known. One example is Os(SCH2)(CO)2(PPh3)2.[3]

Synthesis of a tungsten thioformaldehyde complex.

Synthesis of a osmium thioformaldehyde complex.
References[]
- ^ Despois, D., "Radio Line Observations of Molecular and Isotopic Species in Comet C/1995 O1 (Hale-Bopp) Implications on the Interstellar Origin of Cometary Ices", Earth, Moon, Planets 1999, 79, 103-124.
- ^ Clouthier, D. J.; Ramsay, D. A. (1983). "The Spectroscopy of Formaldehyde and Thioformaldehyde". Annual Review of Physical Chemistry. 34: 31–58. Bibcode:1983ARPC...34...31C. doi:10.1146/annurev.pc.34.100183.000335.
- ^ Schenk, Wolfdieter A. (2011). "The coordination chemistry of small sulfur-containing molecules: A personal perspective". Dalton Trans. 40 (6): 1209–1219. doi:10.1039/C0DT00975J. PMID 21088787.
Categories:
- Thioaldehydes