Triazavirin
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII |
|
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.217.074 |
| Chemical and physical data | |
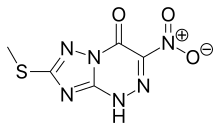
| Formula | C5H4N6O3S |
| Molar mass | 228.189 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Triazavirin (TZV, Riamilovir) is a broad-spectrum antiviral drug developed in Russia through a joint effort of Ural Federal University, Russian Academy of Sciences, Ural Center for Biopharma Technologies and Medsintez Pharmaceutical.[1] It has a novel triazolotriazine core, which represents a new structural class of non-nucleoside antiviral drugs.[2]
The main principle action of triazavirin is to inhibit the synthesis of viral ribonucleic acid (RNA) and the replication of viral genomic fragments through its synthetic analogue to the bases of purine nucleosides.[3][4][5]
Uses[]
It was originally developed as a potential treatment for pandemic influenza strains such as H5N1, and most of the testing that has been done has focused on its anti-influenza activity.[5][3][6][7][8] However, triazavirin has also been found to have antiviral activity against a number of other viruses including Tick-borne encephalitis virus,[4] and Forest-Spring Encephalitis virus,[9] and is also being investigated for potential application against a lethal influenza infection and secondary bacterial pneumonia following influenza,[10] Lassa fever and Ebola virus disease.[11][12][13][14][15] Triazavirin has passed clinical trials and has shown antiviral activity against ARVI.[16][17][18][19] In 2020, testing of triazavirin was started against SARS-CoV-2 in Russia, China, and South Africa. [20][21][22][23][24][25]
In August 2014, the Ministry of Health of Russia issued a registration certificate for triazavirin.[26] The active substance of the drug triazavirin is a new active molecule,[2] and can be dispensed by prescription. The production of triazavirin is carried out at a modern pharmaceutical enterprise LLC "Plant Medsintez".[1] The registration procedure for triazavirin has begun in the Republic of South Africa.[24]
See also[]
- BCX4430
- Brincidofovir
- Favipiravir
- Umifenovir
References[]
- ^ a b "Home". www.medsintez.com. Retrieved 2021-02-25.
- ^ a b Rusinov VL, Sapozhnikova IM, Ulomskii EN, Medvedeva NR, Egorov VV, Kiselev OI, et al. (2015). "Nucleophilic substitution of nitro group in nitrotriazolotriazines as a model of potential interaction with cysteine-containing proteins". Chemistry of Heterocyclic Compounds. 51 (3): 275–280. doi:10.1007/s10593-015-1695-4. S2CID 83702396.
- ^ a b Karpenko I, Deev S, Kiselev O, Charushin V, Rusinov V, Ulomsky E, et al. (May 2010). "Antiviral properties, metabolism, and pharmacokinetics of a novel azolo-1,2,4-triazine-derived inhibitor of influenza A and B virus replication". Antimicrobial Agents and Chemotherapy. 54 (5): 2017–22. doi:10.1128/AAC.01186-09. PMC 2863629. PMID 20194696.
- ^ a b Loginova SI, Borisevich SV, Rusinov VL, Ulomskiĭ UN, Charushin VN, Chupakhin ON (2014). "[Investigation of Triazavirin antiviral activity against tick-borne encephalitis pathogen in cell culture]". Antibiotiki i Khimioterapiia (in Russian). 59 (1–2): 3–5. PMID 25051708.
- ^ a b Loginova SI, Borisevich SV, Maksimov VA, Bondarev VP, Kotovskaia SK, Rusinov VL, et al. (2007). "[Investigation of triazavirin antiviral activity against influenza A virus (H5N1) in cell culture]". Antibiotiki i Khimioterapiia (in Russian). 52 (11–12): 18–20. PMID 19275052.
- ^ Kiselev OI, Deeva EG, Mel'nikova TI, Kozeletskaia KN, Kiselev AS, Rusinov VL, et al. (2012). "[A new antiviral drug Triazavirin: results of phase II clinical trial]". Voprosy Virusologii (in Russian). 57 (6): 9–12. PMID 23477247.
- ^ Kasianenko, K. V.; Lvov, N. I.; Maltsev, O. V.; Zhdanov, K. V. (9 October 2019). "Nucleoside analogues for the treatment of influenza: History and experience". Journal Infectology (in Russian). 11 (3): 20–26. doi:10.22625/2072-6732-2019-11-3-20-26.
- ^ Sologub, T.V.; Tokin, I.I.; Midikari, A.S.; Tsvetkov, V.V. (2017). "A comparative efficacy and safety of using antiviral drugs in therapy of patients with influenza". Infekcionnye Bolezni (in Russian). 15 (3): 25–32. doi:10.20953/1729-9225-2017-3-25-32.
- ^ Loginova SY, Borisevich SV, Rusinov VL, Ulomsky EN, Charushin VN, Chupakhin ON, Sorokin PV (2015). "[Investigation of Therapeutic Efficacy of Triazavirin Against Experimental Forest-Spring Encephalitis on Albino Mice]". Antibiotiki i Khimioterapiia (in Russian). 60 (7–8): 11–3. PMID 26863736.
- ^ Leneva, Irina A.; Falynskova, Irina N.; Makhmudova, Nailya R.; Glubokova, Ekaterina A.; Kartashova, Nadezhda P.; Leonova, Eugenia I.; Mikhailova, Natalya A.; Shestakova, Irina V. (2018-02-22). "Effect of triazavirine on the outcome of a lethal influenza infection and secondary bacterial pneumonia following influenza in mice". Microbiology Independent Research Journal. 4 (1).
- ^ "Target: Ebola". Pravda. 2014-12-22. Retrieved 18 January 2015.
- ^ "Yekaterinburg pharmacies to sell domestic antiviral drug". Retrieved 18 January 2015.
- ^ Cox S (2014-10-17). "Ebola crisis: Vaccine 'too late' for outbreak. BBC News, 17 October 2014". BBC News.
- ^ Kukil Bora. Russia Will Begin Testing Triazavirin, Used For Lassa Fever, And Other Drugs On Ebola: Health Ministry. International Business Times, 12 November 2014
- ^ Kezina, Darya (12 November 2014). "New antiviral drug from Urals will help fight Ebola and other viruses". Russia Beyond the Headlines.
- ^ Tikhonova, E. P.; Петровна, Тихонова Елена; Kuz'mina, T. Yu; Юрьевна, Кузьмина Татьяна; Andronova, N. V.; Владимировна, Андронова Наталья; Tyushevskaya, O. A.; Анатольевна, Тюшевская Ольга; Elistratova, T. A.; Анатольевна, Елистратова Татьяна; Kuz'min, A. E. (2018-04-15). "Study of effectiveness of antiviral drugs (umifenovir, triazavirin) against acute respiratory viral infections". Kazan Medical Journal (in Russian). 99 (2): 215–223. doi:10.17816/KMJ2018-215. ISSN 2587-9359.
- ^ Verevshchikov, V. K.; Shemyakina, E. K.; Sabitov, A. U.; Khamanova, Yu B. (2019). "The Possibilities of Etiotropic Therapy for Influenza and ARVI with Taking into Account the Period of Hospitalization and the Risk of Developing Secondary Complications". Antibiotics and Chemotherapy. Retrieved 2021-02-25.
- ^ Lioznov, D. A.; Анатольевич, Лиознов Дмитрий; Tokin, I. I.; Иванович, Токин Иван; Zubkova, T. G.; Геннадьевна, Зубкова Татьяна; Sorokin, P. V.; Владимирович, Сорокин Павел (2020-12-15). "The practice of using a domestic antiviral drug in the etiotropic therapy of acute respiratory viral infection". Terapevticheskii Arkhiv (in Russian). 92 (12): 160–164. doi:10.26442/00403660.2020.12.200427. ISSN 2309-5342. PMID 33720589.
- ^ Verevshchikov, V. K.; Shemyakina, E. K.; Sabitov, A. U.; Batskalevich, N. A. (2018). "Modern Etiotropic Therapy of Influenza and ARVI in Adult Patients with Premorbid Pathology". Antibiotics and Chemotherapy. Retrieved 2021-02-25.
- ^ Jamshaid U (4 February 2020). "China Testing Russia's Triazavirin As Coronavirus Treatment". Russian Health Ministry. Urdupoint.
- ^ "China Tests Russian Antiviral Drug Which Might Treat Coronavirus As Moscow Warns Of Possible 'Mass Outbreak'". 2020-02-05. Retrieved 11 February 2020.
- ^ Wu, Xiaoke; Yu, Kaijiang; Wang, Yongchen; Xu, Wanhai; Ma, Hongli; Hou, Yan; Li, Yue; Cai, Benzhi; Zhu, Liying; Zhang, Min; Hu, Xiaoli; Gao, Jingshu; Wang, Yu; Qin, Huichao; Wang, Wenjie; Zhao, Mingyan; Wu, Xia; Zhang, Yong; Li, Lu; Li, Kang; Du, Zhimin; Mol, Ben Willem J.; Yang, Baofeng (2020). "Efficacy and safety of triazavirin therapy for coronavirus disease 2019: A pilot randomized controlled trial". Engineering. 6 (10): 1185–1191. doi:10.1016/j.eng.2020.08.011. ISSN 2095-8099. PMC 7476906. PMID 32923016. S2CID 221520816.
- ^ Brandt, Kevin. "SA study to trial antiviral Triazavirin as COVID-19 treatment". ewn.co.za. Retrieved 2021-02-25.
- ^ a b «Discussion on Russia's antiviral drug Triazavirin». SABC News. 21 January 2021. https://www.youtube.com/watch?v=qbMpopd7N14&t=22s
- ^ Sabitov, A. U.; Belousov, V. V.; Edin, A. S.; Oleinichenko, E. V.; Gladunova, E. P.; Tikhonova, E. P.; Kuzmina, T. Yu.; Kalinina, Yu. S.; Sorokin, P. V. (21 November 2020). "Practical Experience of Using Riamilovir in Treatment of Patients with Moderate COVID-19". Antibiotics and Chemotherapy. 65 (7–8): 27–30. doi:10.37489/0235-2990-2020-65-7-8-27-30.
- ^ State Register of Medicines of the Russian Federation http://grls.rosminzdrav.ru/Grls_View_v2.aspx?routingGuid=d552450f-6223-43a2-87af-e5840a632b19&t=
- Antiviral drugs
- Experimental drugs
- Lactams
- Thioethers
- Nitrogen heterocycles
- Heterocyclic compounds with 2 rings
- Russian drugs
- Russian inventions
- Triazoles
- Antiinfective agent stubs