Benzyl iodide
| |
| Names | |
|---|---|
| Preferred IUPAC name
(Iodomethyl)benzene | |
| Other names
Fraissite, iodotoluol, α-iodotoluene, phenylmethyliodide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.009.659 |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| Properties | |
| C7H7I | |
| Molar mass | 218.037 g·mol−1 |
| Appearance | Low-melting crystals or colorless liquid |
| Melting point | 24.5 °C |
| Boiling point | 218 °C (424 °F; 491 K) |
| Insoluble | |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| Flash point | 86 °C (187 °F; 359 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
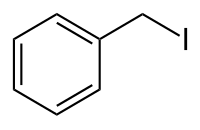
Benzyl iodide is an organic compound with the chemical formula C
7H
7I.[1][2] The compound consists of a benzene ring with an attached iodidemethyl group. The substance is an alkyl halide and is a constitutional isomer of the iodotoluenes.
Synthesis[]
Benzyl iodide can be obtained via the Finkelstein reaction from benzyl chloride and sodium iodide in acetone.

Synthesis of benzyl iodide by Finkelstein reaction
Properties[]
Benzyl iodide forms colorless to yellow needles, melting at 24.5 °C.[3] As a liquid, the compound has the high refractive index of 1.6334. Benzyl iodide is also a powerful lachrymator.[4][5]
See also[]
References[]
- ^ "BENZYL IODIDE". chemicalbook.com. Retrieved 8 June 2017.
- ^ "Benzyl iodide". NIST. webbook.nist.gov. Retrieved 8 June 2017.
- ^ CRC Handbook of Chemistry and Physics, 90. Edition, CRC Press, Boca Raton, Florida, 2009, ISBN 978-1-4200-9084-0, Section 3, Physical Constants of Organic Compounds, p. 3-306.
- ^ Bauta, William E. (2001). "Benzyl Iodide". Encyclopedia of Reagents for Organic Synthesis. onlinelibrary.wiley.com. doi:10.1002/047084289X.rb060. ISBN 978-0471936237.
- ^ Fieser, Louis F.; Fieser, Mary (1982). Organische Chemie (in German). ISBN 978-3-527-25075-2.
Categories:
- Organoiodides
- Benzyl compounds
