Mustard gas

| |

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Chloro-2-[(2-chloroethyl)sulfanyl]ethane | |
| Other names
Bis(2-chloroethyl) sulfide
HD Iprit Schwefel-LOST Lost Sulfur mustard Senfgas Yellow cross liquid Yperite Distilled mustard Mustard T- mixture 1,1'-thiobis[2-chloroethane] Dichlorodiethyl sulfide | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.209.973 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
Chemical formula
|
C4H8Cl2S |
| Molar mass | 159.07 g·mol−1 |
| Appearance | Colorless if pure. Normally ranges from pale yellow to dark brown. Slight garlic or horseradish type odor.[1] |
| Density | 1.27 g/mL, liquid |
| Melting point | 14.4 °C (57.9 °F; 287.5 K) |
| Boiling point | 217 °C (423 °F; 490 K) begins to decompose at 217 °C (423 °F) and boils at 218 °C (424 °F) |
| Negligible | |
| Solubility | soluble in ether, benzene, lipids, alcohol, THF |
| Hazards | |
| Main hazards | Poison, contact hazard, inhalation hazard, corrosive, environmental hazard, carcinogenic, possibly mutagenic |
| Safety data sheet | External MSDS |
EU classification (DSD) (outdated)
|
Very toxic (T+) Dangerous for the environment (N) Vesicant Carc. Cat 1 |
| NFPA 704 (fire diamond) | 
4
1
1 |
| Flash point | 105 °C (221 °F; 378 K) |
| Related compounds | |
Related compounds
|
Nitrogen mustard, Bis(chloroethyl) ether |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Mustard gas or sulfur mustard is a chemical compound belonging to the sulfur-based family of cytotoxic and blister agent chemical warfare agents known as sulfur-mustards or mustard agents. Related chemical compounds with similar chemical structures and similar properties form a class of compounds known collectively as sulfur mustards or mustard agents. The name mustard gas is widely used, but it is technically incorrect: the substance does not actually vaporize into a gas, but instead disperses as a fine mist of liquid droplets.[2]
Mustard gas has a long history of being used as a blister-agent in warfare and, along with organoarsenic compounds such as Lewisite, is the most well-studied of such lethal agents. Mustard gas can form large blisters on exposed skin and in the lungs, often resulting in prolonged illness ending in death.[3] Pure sulfur mustards are colorless, viscous liquids at room temperature. When used in impure forms, such as warfare agents, they are usually yellow-brown and have an odor resembling mustard plants, garlic, or horseradish, hence the name.[2]
As a chemical weapon, mustard gas was first used in World War I, and has been used in several armed conflicts since then, including the Iran–Iraq War. Mustard agents are regulated under the 1993 Chemical Weapons Convention. Three classes of chemicals are monitored under this Convention, with sulfur and nitrogen mustard grouped in Schedule 1, as substances with no use other than in chemical warfare (though since then, mustard gas has been found to be useful in cancer chemotherapy[4]). Mustard agents could be deployed by means of artillery shells, aerial bombs, rockets, or by spraying from warplanes or other aircraft.
Synthesis and reactions[]
Mustard gas is the organic compound with formula (ClCH2CH2)2S. In the Depretz method, mustard gas is synthesized by treating sulfur dichloride with ethylene:
- SCl2 + 2 C2H4 → (ClCH2CH2)2S
In the Levinstein process, disulfur dichloride is used instead:[5][6]
- 8 S2Cl2 + 16 C2H4 → 8 (ClCH2CH2)2S + S8
In the Meyer method, thiodiglycol is produced from chloroethanol and potassium sulfide and chlorinated with phosphorus trichloride:[7]
- 3 (HOCH2CH2)2S + 2 PCl3 → 3 (ClCH2CH2)2S + 2 P(OH)3
In the Meyer-Clarke method, concentrated hydrochloric acid (HCl) instead of PCl3 is used as the chlorinating agent:
- (HOCH2CH2)2S + 2 HCl → (ClCH2CH2)2S + 2 H2O
Thionyl chloride and phosgene, the latter of which (CG) is also a choking agent, have also been used as chlorinating agents, with the added possibility of both agents producing additional mechanisms of toxicity if they remain as impurities in the finished product.
Mustard gas is a viscous liquid at normal temperatures. The pure compound has a melting point of 14 °C (57 °F) and decomposes before boiling at 218 °C (424 °F).
Reaction of mustard gas with sodium ethoxide gives divinyl sulfide:
- (ClCH2CH2)2S + 2 NaOEt → (CH2=CH)2S + 2 EtOH + 2 NaCl
Mustard gas can be readily decontaminated through reaction with chloramine-T.[8]
Mechanism of cellular toxicity[]
The compound readily eliminates a chloride ion by intramolecular nucleophilic substitution to form a cyclic sulfonium ion. This very reactive intermediate tends to cause permanent alkylation of the guanine nucleotide in DNA strands, which prevents cellular division and generally leads directly to programmed cell death,[9] or, if cell death is not immediate, the damaged DNA may lead to the development of cancer.[9] Oxidative stress would be another pathology involved in mustard gas toxicity. Mustard gas is not very soluble in water but is very soluble in fat, contributing to its rapid absorption into the skin.[9]
 Mustard gas (bis(chloroethyl) thioether) alkylating a DNA amine base
Mustard gas (bis(chloroethyl) thioether) alkylating a DNA amine base
In the wider sense, compounds with the structural element BCH2CH2X, where X is any leaving group and B is a Lewis base are known as mustards. Such compounds can form cyclic "onium" ions (sulfonium, ammoniums, etc.) that are good alkylating agents. Examples are bis(2-chloroethyl)ether, the (2-haloethyl)amines (nitrogen mustards), and sulfur sesquimustard, which has two α-chloroethyl thioether groups (ClH2CCH2S−) connected by an ethylene (−CH2CH2−) group.[citation needed] These compounds have a similar ability to alkylate DNA, but their physical properties, e.g. melting points, may vary.
Physiological effects[]
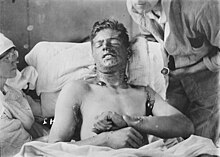
The mustard agent has extremely powerful vesicant (blistering) effects on its victims. In addition, it is strongly mutagenic and carcinogenic, due to its alkylating properties. It is also lipophilic. Because people exposed to mustard agents rarely suffer immediate symptoms, and mustard-contaminated areas may appear completely normal, victims can unknowingly receive high dosages. Within 24 hours of exposure to the mustard agent, victims experience intense itching and skin irritation, which gradually turns into large blisters filled with yellow fluid wherever the mustard agent contacted the skin. These are chemical burns and are very debilitating. Mustard agent vapor easily penetrates clothing fabrics such as wool or cotton, so it is not only the exposed skin of victims that get burned. If the victim's eyes were exposed then they become sore, starting with conjunctivitis (also known as pink eye), after which the eyelids swell, resulting in temporary blindness. In rare cases of extreme ocular exposure to mustard gas vapors, corneal ulceration, anterior chamber scarring, and neovascularization have occurred. In these severe and infrequent cases, corneal transplantation has been used as a treatment option.[10] Miosis, when the pupil constricts more than usual, may also occur, which is probably the result of the cholinomimetic activity of mustard.[11] At very high concentrations, if inhaled, mustard agent causes bleeding and blistering within the respiratory system, damaging mucous membranes and causing pulmonary edema. Depending on the level of contamination, mustard agent burns can vary between first and second degree burns, though they can also be every bit as severe, disfiguring and dangerous as third degree burns.[12] Severe mustard agent burns (i.e. where more than 50% of the victim's skin has been burned) are often fatal, with death occurring after days or even weeks have passed. Mild or moderate exposure to mustard agent is unlikely to kill, though victims require lengthy periods of medical treatment and convalescence before recovery is complete.
The mutagenic and carcinogenic effects of mustard agent mean that victims who recover from mustard agent burns have an increased risk of developing cancer in later life. In a study of patients 25 years after wartime exposure to chemical weaponry, c-DNA microarray profiling indicated that 122 genes were significantly mutated in the lungs and airways of mustard gas victims. Those genes all correspond to functions commonly affected by mustard gas exposure, including apoptosis, inflammation, and stress responses.[13]

The vesicant property of mustard agent can be neutralized by oxidation or chlorination, using household bleach (sodium hypochlorite), or by nucleophilic attack using e.g. decontamination solution "DS2" (2% NaOH, 70% diethylenetriamine, 28% 2-methoxyethanol). After initial decontamination of the victim's wounds is complete, medical treatment is similar to that required by any conventional burn. The degree of pain and discomfort suffered by the victim is also comparable. Mustard agent burns heal slowly, and, as with other types of burn, present a risk of sepsis caused by pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa. The mechanisms behind mustard gas's effect on endothelial cells are still being studied, but recent studies have shown that high levels of exposure can induce high rates of both necrosis and apoptosis. In vitro tests have shown that at low concentrations of mustard gas, where apoptosis is the predominant result of exposure, pretreatment with 50 mM N-acetyl-L-cysteine (NAC) was able to decrease the rate of apoptosis. NAC protects actin filaments from reorganization by mustard gas, demonstrating that actin filaments play a large role in the severe burns observed in victims.[14]
A British nurse treating soldiers with mustard agent burns during World War I commented:[15]

They cannot be bandaged or touched. We cover them with a tent of propped-up sheets. Gas burns must be agonizing because usually the other cases do not complain, even with the worst wounds, but gas cases are invariably beyond endurance and they cannot help crying out.
Formulations[]
In its history, various types and mixtures of mustard gas have been employed. These include:
- H – Also known as HS ("Hun Stuff") or Levinstein mustard. This is named after the inventor of the quick but dirty Levinstein Process for manufacture,[5][6] reacting dry ethylene with sulfur monochloride under controlled conditions. Undistilled mustard gas contains 20–30% impurities, for which reason it does not store as well as HD. Also, as it decomposes, it increases in vapor pressure, making the munition it is contained in likely to split, especially along a seam, releasing the agent to the atmosphere [1]
- HD – Codenamed Pyro by the British, and Distilled Mustard by the US.[1] Distilled sulfur mustard (bis(2-chloroethyl) sulfide); approximately 96% pure. The term "mustard gas" usually refers to this variety of sulfur mustard. A much-used path of synthesis was based upon the reaction of thiodiglycol with hydrochloric acid.
- HT – Codenamed Runcol by the British, and Mustard T- mixture by the US.[1] A mixture of 60% mustard gas (HD) and 40% T (bis[2-(2-chloroethylthio)ethyl] ether), a related vesicant with lower freezing point, lower volatility and similar vesicant characteristics.
- HL – A blend of distilled mustard (HD) and Lewisite (L), originally intended for use in winter conditions due to its lower freezing point compared to the pure substances. The Lewisite component of HL was used as a form of antifreeze.[16]
- HQ – A blend of distilled mustard (HD) and sesquimustard (Q) (Gates and Moore 1946).
Mustard gas agents (class)[]
The complete list of effective mustard gas agents commonly stockpiled is as follows:[citation needed]
| Chemical | Code | Trivial name | CAS number | PubChem | Structure |
|---|---|---|---|---|---|
| Bis(2-chloroethyl)sulfide | H/HD | Mustard | 505-60-2 | CID 10461 from PubChem | |
| 1,2-Bis-(2-chloroethylthio)-ethane | Q | Sesquimustard | 3563-36-8 | CID 19092 from PubChem | |
| Bis-(2-chloroethylthioethyl)-ether | T | O-Mustard | 63918-89-8 | CID 45452 from PubChem | |
| 2-Chloroethyl chloromethyl sulfide | 2625-76-5 | ||||
| Bis-(2-chloroethylthio)-methane | HK | 63869-13-6 | |||
| Bis-1,3-(2-chloroethylthio)-n-propane | 63905-10-2 | ||||
| Bis-1,4-(2-chloroethylthio)-n-butane | 142868-93-7 | ||||
| Bis-1,5-(2-chloroethylthio)-n-pentane | 142868-94-8 | ||||
| Bis-(2-chloroethylthiomethyl)-ether | 63918-90-1 |
History[]
Development[]
Mustard agent was possibly developed as early as 1822 by César-Mansuète Despretz (1798–1863).[17] Despretz described the reaction of sulfur dichloride and ethylene but never made mention of any irritating properties of the reaction product. In 1854, another French chemist, Alfred Riche (1829–1908), repeated this procedure, also without describing any adverse physiological properties. In 1860, the British scientist Frederick Guthrie synthesized and characterized the mustard agent compound and noted its irritating properties, especially in tasting.[18] Also in 1860, chemist Albert Niemann, known as a pioneer in cocaine chemistry, repeated the reaction, and recorded blister-forming properties. In 1886, Viktor Meyer published a paper describing a synthesis that produced good yields. He combined 2-chloroethanol with aqueous potassium sulfide, and then treated the resulting thiodiglycol with phosphorus trichloride. The purity of this compound was much higher and consequently the adverse health effects upon exposure were much more severe. These symptoms presented themselves in his assistant, and in order to rule out the possibility that his assistant was suffering from a mental illness (psychosomatic symptoms), Meyer had this compound tested on laboratory rabbits, most of which died. In 1913, the English chemist Hans Thacher Clarke (known for the Eschweiler-Clarke reaction) replaced the phosphorus trichloride with hydrochloric acid in Meyer's formulation while working with Emil Fischer in Berlin. Clarke was hospitalized for two months for burns after one of his flasks broke. According to Meyer, Fischer's report on this accident to the German Chemical Society sent the German Empire on the road to chemical weapons.[19]
Mustard agent can have the effect of turning a patient's skin different colors, including reds, oranges, pinks, and in unusual cases, blues. The German Empire during World War I relied on the Meyer-Clarke method because 2-chloroethanol was readily available from the German dye industry of that time.
Use[]

Mustard agent was first used effectively in World War I by the German army against British and Canadian soldiers near Ypres, Belgium, in 1917 and later also against the French Second Army. The name Yperite comes from its usage by the German army near the town of Ypres. The Allies did not use mustard agent until November 1917 at Cambrai, France, after the armies had captured a stockpile of German mustard shells. It took the British more than a year to develop their own mustard agent weapon, with production of the chemicals centred on Avonmouth Docks (the only option available to the British was the Despretz–Niemann–Guthrie process).[20][21] This was used first in September 1918 during the breaking of the Hindenburg Line.
Mustard gas was originally assigned the name LOST, after the scientists Wilhelm Lommel and Wilhelm Steinkopf, who developed a method of large-scale production for the Imperial German Army in 1916.[22]
Mustard agent was dispersed as an aerosol in a mixture with other chemicals, giving it a yellow-brown color and a distinctive odor. Mustard agent has also been dispersed in such munitions as aerial bombs, land mines, mortar rounds, artillery shells, and rockets.[1] Exposure to mustard agent was lethal in about 1% of cases. Its effectiveness was as an incapacitating agent. The early countermeasures against mustard agent were relatively ineffective, since a soldier wearing a gas mask was not protected against absorbing it through his skin and being blistered. A common countermeasure was using a urine-soaked mask or facecloth to prevent or reduce injury, a readily available remedy attested by soldiers in documentaries (e.g. They Shall Not Grow Old in 2018) and others (such as forward aid nurses) interviewed between 1947 and 1981 by the British Broadcasting Corporation for various World War One history programs; however, the effectiveness of this measure is unclear.
Mustard agent is a persistent weapon that remains on the ground for weeks, and it continues to cause ill effects. If mustard agent contaminates a soldier's clothing and equipment while cold, then the other soldiers or nurses with whom they share an enclosed space could become poisoned as contaminated items warm up enough material to become an airborne toxic agent. An example of this was depicted in a British and Canadian documentary about life in the trenches, particularly once the "sousterrain" (subways and berthing areas underground) were completed in Belgium and France. Towards the end of World War I, mustard agent was used in high concentrations as an area-denial weapon that forced troops to abandon heavily contaminated areas.

Since World War I, mustard agent has been used in several wars and other conflicts, usually against people who cannot retaliate in kind:[23]
- United Kingdom against the Red Army in 1919[24]
- Alleged British use in Mesopotamia in 1920[25]
- Spain and France against the Rifian resistance in Morocco during the Rif War of 1921–27 (see also: Spanish use of chemical weapons in the Rif War)[23][26]
- Italy in Libya in 1930[23]
- The Soviet Union in Xinjiang, Republic of China, during the Soviet Invasion of Xinjiang against the 36th Division (National Revolutionary Army) in 1934, and also in the Xinjiang War (1937) in 1936–37[24][26]
- Italy against Abyssinia (now Ethiopia) in 1935-1936[23]
- The Japanese Empire against China in 1937–1945[24]
- The United States Government against US Naval recruits at The Great Lakes Naval Base, June 3, 1945.[27]
- The 2 December 1943 air raid on Bari destroyed an Allied stockpile of mustard gas on the SS John Harvey.[28]
- Egypt against North Yemen in 1963–1967[23]
- Iraq against Kurds in the town of Halabja during the Halabja chemical attack[24][29]
- Iraq against Iranians in 1983–1988[30]
- Possibly in Sudan against insurgents in the civil war, in 1995 and 1997.[23]
- In the Iraq War, abandoned stockpiles of mustard gas shells were destroyed in the open air,[31] and were used against Coalition forces in roadside bombs.[32]
- By ISIS forces against Kurdish forces in Iraq in August 2015.[33]
- By ISIS against another rebel group in the town of Mare' in 2015.[34]
- According to Syrian State media, by ISIS against Syrian Army during the battle in Deir ez-Zor in 2016.[35]
In 1943, during the Second World War, an American shipment of mustard agent exploded aboard a supply ship that was bombed during an air raid in the harbor of Bari, Italy. Eighty-three of the 628 hospitalized victims who had been exposed to the mustard agent died.[36]
After WWII, stockpiled mustard agent was dumped by the British in the sea near Port Elizabeth, South Africa, resulting in burn cases among trawler crews.[37]
The use of toxic gases or other chemicals, including mustard agent, during warfare is known as chemical warfare, and this kind of warfare was prohibited by the Geneva Protocol of 1925, and also by the later Chemical Weapons Convention of 1993. The latter agreement also prohibits the development, production, stockpiling, and sale of such weapons.
In September 2012 a US official stated that the rebel militant group ISIS was manufacturing and using mustard gas in Syria and Iraq, which was allegedly confirmed by the group's head of chemical weapons development, Sleiman Daoud al-Afari, who has since been captured.[38][39][40]
Development of the first chemotherapy drug[]
As early as 1919 it was known that mustard agent was a suppressor of hematopoiesis.[41] In addition, autopsies performed on 75 soldiers who had died of mustard agent during World War I were done by researchers from the University of Pennsylvania who reported decreased counts of white blood cells.[36] This led the American Office of Scientific Research and Development (OSRD) to finance the biology and chemistry departments at Yale University to conduct research on the use of chemical warfare during World War II.[36][42]
As a part of this effort, the group investigated nitrogen mustard as a therapy for Hodgkin's lymphoma and other types of lymphoma and leukemia, and this compound was tried out on its first human patient in December 1942. The results of this study were not published until 1946, when they were declassified.[42] In a parallel track, after the air raid on Bari in December 1943, the doctors of the U.S. Army noted that white blood cell counts were reduced in their patients. Some years after World War II was over, the incident in Bari and the work of the Yale University group with nitrogen mustard converged, and this prompted a search for other similar chemical compounds. Due to its use in previous studies, the nitrogen mustard called "HN2" became the first cancer chemotherapy drug, mustine (also called chlormethine), to be used.
Disposal[]
In the United States, storage and incineration of mustard agent and other poison gases was carried out by the U.S. Army Chemical Materials Agency.[43] Disposal projects at the two remaining American chemical weapons sites, will be carried out at their sites near Richmond, Kentucky, and Pueblo, Colorado. Although not yet Declassified,[specify] toxicology specialists who dealt with the accidental puncturing of World War I gas stockpiles add that Air Force bases in Colorado have been made available to assist veterans of the 2003 American war with Iraq in which many Marines were exposed to gas as caches of up to 25,000 lb (11,000 kg).[citation needed] The United Nations definition of a weapon of mass destruction for mustard gas is 30,000 lb (14,000 kg), typically the Marines and other coalition allies discovered caches of 25,000 pounds (11,000 kg) located across a road from 5,000 pounds (2,300 kg) caches as multiple memoirs attest. These were discovered by the assistance of host country allies, or through leaks affecting personnel in an area with a weapon and gas cache called an ASP.
New detection techniques are being developed in order to detect the presence of mustard gas and its metabolites. The technology is portable and detects small quantities of the hazardous waste and its oxidized products, which are notorious for harming unsuspecting civilians. The immunochromatographic assay would eliminate the need for expensive, time-consuming lab tests and enable easy-to-read tests to protect civilians from sulfur-mustard dumping sites.[44]
In 1946, 10,000 drums of mustard gas (2,800 tonnes) stored at the production facility of Stormont Chemicals in Cornwall, Ontario, Canada were loaded onto 187 boxcars for the 900 miles (1,400 km) journey to be buried at sea on board a 400 foot (120 m) long barge 40 miles (64 km) south of Sable Island, southeast of Halifax at a depth of 600 fathoms (1,100 m). The dump location is 42 degrees, 50 minutes north by 60 degrees, 12 minutes west.[45]
A large British stockpile of old mustard agent that had been made and stored since World War I at M. S. Factory, Valley near Rhydymwyn in Flintshire, Wales, was destroyed in 1958.[46]
Most of the mustard gas agent found in Germany after World War II was dumped into the Baltic Sea. Between 1966 and 2002, fishermen have found about 700 chemical weapons in the region of Bornholm, most of which contain mustard gas. One of the more frequently dumped weapons was the "Sprühbüchse 37" (SprüBü37, Spray Can 37, 1937 being the year of its fielding with the German Army). These weapons contain mustard gas mixed with a thickener, which gives it a tar-like viscosity. When the content of the SprüBü37 comes in contact with water, only the mustard gas in the outer layers of the lumps of viscous mustard hydrolyzes, leaving behind amber-colored residues that still contain most of the active mustard gas. On mechanically breaking these lumps (e.g., with the drag board of a fishing net or by the human hand) the enclosed mustard gas is still as active as it had been at the time the weapon was dumped. These lumps, when washed ashore, can be mistaken for amber, which can lead to severe health problems. Artillery shells containing mustard gas and other toxic ammunition from World War I (as well as conventional explosives) can still be found in France and Belgium. These were formerly disposed of by explosion undersea, but since the current environmental regulations prohibit this, the French government is building an automated factory to dispose of the accumulation of chemical shells.
In 1972, the U.S. Congress banned the practice of disposing of chemical weapons into the ocean by the United States. 29,000 tons of nerve and mustard agents had already been dumped into the ocean off the United States by the U.S. Army. According to a report created in 1998 by William Brankowitz, a deputy project manager in the U.S. Army Chemical Materials Agency, the army created at least 26 chemical weapons dumping sites in the ocean offshore from at least 11 states on both the East Coast and the West Coast (in Operation CHASE, Operation Geranium, etc.). In addition, due to poor recordkeeping, about one-half of the sites have only their rough locations known.[47]
In June 1997, India declared its stock of chemical weapons of 1,044 tonnes (1,151 short tons) of mustard gas.[48][49] By the end of 2006, India had destroyed more than 75 percent of its chemical weapons/material stockpile and was granted extension for destroying the remaining stocks by April 2009 and was expected to achieve 100 percent destruction within that time frame.[48] India informed the United Nations in May 2009 that it had destroyed its stockpile of chemical weapons in compliance with the international Chemical Weapons Convention. With this India has become the third country after South Korea and Albania to do so.[50][51] This was cross-checked by inspectors of the United Nations.
Producing or stockpiling mustard gas is prohibited by the Chemical Weapons Convention. When the convention entered force in 1997, the parties declared worldwide stockpiles of 17,440 tonnes of mustard gas. As of December 2015, 86% of these stockpiles had been destroyed.[52]
A significant portion of the stockpile of mustard agent in the United States was stored at the Edgewood Area of Aberdeen Proving Ground in Maryland. Approximately 1,621 tons of mustard agent were stored in one-ton containers on the base under heavy guard. A chemical neutralization plant was built on the proving ground and neutralized the last of this stockpile in February 2005. This stockpile had priority because of the potential for quick reduction of risk to the community. The nearest schools were fitted with overpressurization machinery to protect the students and faculty in the event of a catastrophic explosion and fire at the site. These projects, as well as planning, equipment, and training assistance, were provided to the surrounding community as a part of the Chemical Stockpile Emergency Preparedness Program (CSEPP), a joint program of the Army and the Federal Emergency Management Agency (FEMA).[53] Unexploded shells containing mustard agent and other chemical agents are still present in several test ranges in proximity to schools in the Edgewood area, but the smaller amounts of poison gas (4 to 14 pounds (1.8 to 6.4 kg)) present considerably lower risks. These remnants are being detected and excavated systematically for disposal. The U.S. Army Chemical Materials Agency oversaw disposal of several other chemical weapons stockpiles located across the United States in compliance with international chemical weapons treaties. These include the complete incineration of the chemical weapons stockpiled in Alabama, Arkansas, Indiana, and Oregon. Earlier, this agency had also completed destruction of the chemical weapons stockpile located on Johnston Atoll located south of Hawaii in the Pacific Ocean.[54] The largest mustard agent stockpile, of about 6,196 tons, was stored at the Deseret Chemical Depot in northern Utah. The incineration of this stockpile began in 2006. In May 2011, the last one-ton tank of mustard agent was incinerated at the Deseret Chemical Depot, and the last mustard agent artillery shells at Deseret were incinerated in January 2012.
In 2008, many empty mustard agent aerial bombs were found in an excavation at the Marrangaroo Army Base just west of Sydney, Australia.[55][56] In 2009, a mining survey near Chinchilla, Queensland, uncovered 144 105-millimeter howitzer shells, some containing "Mustard H", that had been buried by the U.S. Army during World War II.[56][57]
In 2014, a collection of 200 bombs was found on the boundary between the Flemish villages of Passendale and Moorslede. The majority of the bombs were filled with mustard agent. The bombs are a leftover from the German army and were meant to be used in the Battle of Passchendale in World War I. It was the largest collection of chemical weapons ever found in Belgium.[58]
Post-War accidental exposure[]
In 2002, an archaeologist at the Presidio Trust archaeology lab in San Francisco was exposed to mustard agent, which had been dug up at the Presidio of San Francisco, a former military base.[59]
In 2010, a clamming boat pulled up some old artillery shells of World War I from the Atlantic Ocean south of Long Island, New York. Multiple fishermen suffered from skin blistering and respiratory irritation severe enough to require their hospitalization.[60]
WWII-era tests on men[]
From 1943 to 1944, mustard agent experiments were performed on Australian service volunteers in tropical Queensland, Australia, by British Army and American experimenters, resulting in some severe injuries. One test site, the Brook Islands National Park, was chosen to simulate Pacific islands held by the Imperial Japanese Army.[61][62]

The United States tested mustard gas and other chemical agents including nitrogen mustard and lewisite on up to 60,000 servicemen during and after WWII. The experiments were classified secret and as with Agent Orange, claims for medical care and compensation were routinely denied, even after the WWII-era tests were declassified in 1993. The Department of Veterans Affairs stated that it would contact 4,000 surviving test subjects but failed to do so, eventually only contacting 600. Skin cancer, severe eczema, leukemia, and chronic breathing problems plagued the test subjects, some of whom were as young as 19 at the time of the tests, until their deaths, but even those who had previously filed claims with the VA went without compensation.[63]

African-American servicemen were tested alongside white men in separate trials to determine whether their skin color would afford them a degree of immunity to the agents, and Nisei servicemen, some of whom had joined after their release from Japanese American Internment Camps were tested to determine susceptibility of Japanese military personnel to these agents. These tests also included Puerto-Rican subjects.[64]
Detection in biological fluids[]
Urinary concentrations of the thiodiglycol hydrolysis products of mustard gas have been used to confirm a diagnosis of chemical poisoning in hospitalized victims. The presence in urine of 1,1'-sulfonylbismethylthioethane (SBMTE), a conjugation product with glutathione, is considered a more specific marker, since this metabolite is not found in specimens from unexposed persons. Intact mustard gas was detected in postmortem fluids and tissues of a man who died one week post-exposure.[65]
See also[]
- Bis(chloromethyl) ether
- Blister agent
- Chlorine gas
- Half mustard
- Lewisite
- Nitrogen mustard
- Phosgene oxime
- Poison gas in World War I
- Selenium mustard
- Yellow Cross (chemical warfare)
References[]
- ^ Jump up to: a b c d e FM 3–8 Chemical Reference handbook, US Army, 1967
- ^ Jump up to: a b https://www.acs.org/content/dam/acsorg/education/resources/highschool/chemmatters/gc-mustard-gas-personal-safety-and-natl-security.pdf
- ^ See:
- Mustard gas (Sulphur Mustard) (IARC Summary & Evaluation, Supplement7, 1987). Inchem.org (1998-02-09). Retrieved on 2011-05-29.
- Institute of Medicine (1993). "History and Analysis of Mustard Agent and Lewisite Research Programs in the United States". Veterans at Risk: The Health Effects of Mustard Gas and Lewisite. ISBN 978-0-309-04832-3.
- "CDC - Facts About Sulfur Mustard". cdc.gov. Archived from the original on 2006-08-09.
- "NATO Presses New Libyan Leaders to Eliminate Mustard Agent - Global Security Newswire - NTI". NTI: Nuclear Threat Initiative.
- ^ Smith, Susan L. (27 February 2017). "War! What is it good for? Mustard gas medicine". CMAJ. 189 (8): E321–E322. doi:10.1503/cmaj.161032. PMC 5325736. PMID 28246228.
- ^ Jump up to: a b Stewart, Charles D. (2006). Weapons of mass casualties and terrorism response handbook. Boston: Jones and Bartlett. p. 47. ISBN 0-7637-2425-4.
- ^ Jump up to: a b "Chemical Weapons Production and Storage". Federation of American Scientists. Archived from the original on August 11, 2014.
- ^ Institute of Medicine (1993). Chapter 5: Chemistry of Sulfur Mustard and Lewisite. Veterans at Risk: The Health Effects of Mustard Gas and Lewisite. The National Academies Press. ISBN 0-309-04832-X.
- ^ Yasukazu Ura; Gozyo Sakata (2007), "Chloroamines", Ullmann's Encyclopedia of Industrial Chemistry (7th ed.), Wiley, p. 5
- ^ Jump up to: a b c Mustard agents: description, physical and chemical properties, mechanism of action, symptoms, antidotes and methods of treatment. Organisation for the Prohibition of Chemical Weapons. Accessed June 8, 2010.
- ^ Safarinejad, M. R.; Moosavi, S. A.; Montazeri, B (2001). "Ocular injuries caused by mustard gas: diagnosis, treatment, and medical defense". Military Medicine. 166 (1): 67–70. doi:10.1093/milmed/166.1.67. PMID 11197102.
- ^ Vesicants. brooksidepress.org
- ^ Effects of mustard gas, WW1|Gas Warfare Medical Aspects|World War II Resource Centre. Vlib.us (2004-08-23). Retrieved on 2011-05-29.
- ^ Najafi, Ali; Masoudi-Nejad, Ali; Imani Fooladi, Abbas Ali; Ghanei, Mostafa; Nourani, Mohamad Reza (2014). "Microarray gene expression analysis of the human airway in patients exposed to sulfur mustard". Journal of Receptors and Signal Transduction. 34 (4): 283–9. doi:10.3109/10799893.2014.896379. PMID 24823320. S2CID 41665583.
- ^ Dabrowska, Milena I.; Becks, Lauren L.; Lelli, Jr., Joseph L.; Levee, Minette G.; Hinshaw, Daniel B. (1996). "Sulfur Mustard Induces Apoptosis and Necrosis in Endothelial Cells". Toxicology and Applied Pharmacology. 141 (2): 568–83. doi:10.1006/taap.1996.0324. PMID 8975783.
- ^ Van Bergen, Leo (2009). Before My Helpless Sight: Suffering, Dying and Military Medicine on the Western Front, 1914–1918. Ashgate Publishing, Ltd. p. 184. ISBN 978-0-7546-5853-5.
- ^ The Emergency Response Safety and Health Database: Mustard-Lewisite Mixture (HL). National Institute for Occupational Safety and Health. Accessed March 19, 2009.
- ^ By Any Other Name: Origins of Mustard Gas Archived 2014-02-01 at the Wayback Machine. Itech.dickinson.edu (2008-04-25). Retrieved on 2011-05-29.
- ^ F. Guthrie (1860). "XIII.—On some derivatives from the olefines". Q. J. Chem. Soc. 12 (1): 109–126. doi:10.1039/QJ8601200109.
- ^ Duchovic, Ronald J.; Vilensky, Joel A. (2007). "Mustard Gas: Its Pre-World War I History". J. Chem. Educ. 84 (6): 944. Bibcode:2007JChEd..84..944D. doi:10.1021/ed084p944.
- ^ Edited by David Large. The Port of Bristol, 1848-1884.CS1 maint: extra text: authors list (link)
- ^ "Photographic Archive of Avonmouth Bristol BS11". BristolPast.co.uk. Archived from the original on July 3, 2011. Retrieved 12 May 2014.
- ^ Fischer, Karin (June 2004). Schattkowsky, Martina (ed.). Steinkopf, Georg Wilhelm, in: Sächsische Biografie (in German) (Online ed.). Institut für Sächsische Geschichte und Volkskunde. Retrieved 2010-12-28.
- ^ Jump up to: a b c d e f Blister Agent: Mustard gas (H, HD, HS) Archived July 24, 2007, at the Wayback Machine, CBWinfo.com
- ^ Jump up to: a b c d Pearson, Graham S. "Uses of CW since the First World War". Federation of American Scientitst. Archived from the original on August 22, 2010. Retrieved 2010-06-28.
- ^ Townshend, Charles (1986). "Civilisation and "Frightfulness": Air Control in the Middle East Between the Wars". In Chris Wrigley (ed.). Warfare, diplomacy and politics: essays in honour of A.J.P. Taylor. Hamilton. p. 148. ISBN 978-0-241-11789-7.
- ^ Jump up to: a b Daniel Feakes (2003). "Global society and biological and chemical weapons" (PDF). In Mary Kaldor; Helmut Anheier; Marlies Glasius (eds.). Global Civil Society Yearbook 2003. Oxford University Press. pp. 87–117. ISBN 0-19-926655-7. Archived from the original (PDF) on 2007-07-11.
- ^ "The Tox Lab: When U Chicago Was in the Chemical Weapons "Business" | Newcity". 2013-09-23. Retrieved 2021-07-02.
- ^ K. Coleman (23 May 2005). A History of Chemical Warfare. Palgrave Macmillan UK. pp. 74–. ISBN 978-0-230-50183-6.
- ^ Lyon, Alistair (2008-07-09). "Iran's Chemical Ali survivors still bear scars". Reuters. Retrieved 2008-11-17.
- ^ Benschop, Hendrik P.; van der Schans, Govert P.; Noort, Daan; Fidder, Alex; Mars-Groenendijk, Roos H.; de Jong, Leo P.A. (1 July 1997). "Verification of Exposure to Sulfur Mustard in Two Casualties of the Iran-Iraq Conflict". Journal of Analytical Toxicology. 21 (4): 249–251. doi:10.1093/jat/21.4.249. PMID 9248939.
- ^ "More Than 600 Reported Chemical Exposure in Iraq, Pentagon Acknowledges". The New York Times. 6 Nov 2014.
- ^ "Veterans Hurt by Chemical Weapons in Iraq Get Apology". The New York Times. 25 Mar 2015.
- ^ Deutsch, Anthony (15 February 2016). "Samples confirm Islamic State used mustard gas in Iraq - diplomat". Reuters. Retrieved 15 February 2016.
- ^ Deutsch, Anthony (2015-11-06). "Chemical weapons used by fighters in Syria—sources". Reuters. Retrieved 2017-06-30.
- ^ "Syria war: IS 'used mustard gas' on Assad troops". BBC News. 2016-04-05. Retrieved 2017-06-30.
- ^ Jump up to: a b c Faguet, Guy B. (2005). The War on Cancer. Springer. p. 71. ISBN 1-4020-3618-3.
- ^ "NEWSLETTER - JUNE 1992 NEWSLETTER - Johannesburg - South African Military History Society - Title page". Samilitaryhistory.org. Retrieved 2013-08-23.
- ^ Paul Blake (11 September 2015). "US official: 'IS making and using chemical weapons in Iraq and Syria'". BBC. Retrieved 16 September 2015.
- ^ Lizzie Dearden (11 September 2015). "Isis 'manufacturing and using chemical weapons' in Iraq and Syria, US official claims". The Independent. Retrieved 16 September 2015.
- ^ Jamie Schram (9 March 2016). "Captured ISIS head of chemical weapons says they've got mustard gas'". NYPost. Retrieved 9 March 2016.
- ^ Krumbhaar EB (1919). "Rôle of the blood and the bone marrow in certain forms of gas poisoning: I. peripheral blood changes and their significance". JAMA. 72: 39–41. doi:10.1001/jama.1919.26110010018009f.
- ^ Jump up to: a b Gilman A (May 1963). "The initial clinical trial of nitrogen mustard". Am. J. Surg. 105 (5): 574–8. doi:10.1016/0002-9610(63)90232-0. PMID 13947966.
- ^ The U.S. Army's Chemical Materials Agency (CMA) Archived October 15, 2004, at the Wayback Machine. cma.army.mil. Retrieved on November 11, 2011.
- ^ Sathe, Manisha; Srivastava, Shruti; Merwyn, S.; Agarwal, G. S.; Kaushik, M. P. (24 July 2014). "Competitive immunochromatographic assay for the detection of thiodiglycol sulfoxide, a degradation product of sulfur mustard". The Analyst. 139 (20): 5118–26. Bibcode:2014Ana...139.5118S. doi:10.1039/C4AN00720D. PMID 25121638.
- ^ "Hill 70 & Cornwall's Deadly Mustard Gas Plant". Cornwall Community Museum. Stormont, Dundas and Glengarry Historical Society. 18 September 2016. Retrieved 23 December 2016.
- ^ "Valley Factory, Rhydymwyn". 24 July 2010.
- ^ Bull, John (30 October 2005). "The Deadliness Below". Daily Press Virginia. Archived from the original on 2012-07-23. Retrieved 2013-01-28.
- ^ Jump up to: a b "India to destroy chemical weapons stockpile by 2009". Dominican Today. Archived from the original on 7 September 2013. Retrieved 30 April 2013.
- ^ Smithson, Amy Gaffney, Frank, Jr.; 700+ words. "India declares its stock of chemical weapons". Archived from the original on 6 November 2012. Retrieved 30 April 2013.CS1 maint: multiple names: authors list (link)
- ^ "Zee News – India destroys its chemical weapons stockpile". Zeenews.india.com. 14 May 2009. Retrieved 30 April 2013.
- ^ "Archived copy". Archived from the original on 21 May 2009. Retrieved 20 May 2009.CS1 maint: archived copy as title (link)
- ^ Organisation for the Prohibition of Chemical Weapons (30 November 2016). "Annex 3". Report of the OPCW on the Implementation of the Convention on the Prohibition of the Development, Production, Stockpiling and Use of Chemical Weapons and on Their Destruction in 2015 (Report). p. 42. Retrieved 8 March 2017.
- ^ "CSEPP Background Information". US Federal Emergency Management Agency (FEMA). 2 May 2006. Archived from the original on 27 May 2006.
- ^ "Milestones in U.S. Chemical Weapons Storage and Destruction, fact sheet, US Chemical Materials Agency". Archived from the original on 15 September 2012. Retrieved 15 January 2012.
- ^ Ashworth L (7 August 2008). "Base's phantom war reveals its secrets". Fairfax Digital. Archived from the original on 5 December 2008.
- ^ Jump up to: a b Chemical Warfare in Australia. Mustardgas.org. Retrieved on 29 May 2011.
- ^ Cumming, Stuart (11 November 2009). "Weapons await UN inspection". Toowoomba Chronicle.
- ^ "Farmer discovers 200 bombs (Dutch)". 5 March 2014.
- ^ Sullivan, Kathleen (2002-10-22). "Vial found in Presidio may be mustard gas / Army experts expected to identify substance". sfgate.com.
- ^ Wickett, Shana; Beth Daley (2010-06-08). "Fishing crewman exposed to mustard gas from shell". The Boston Globe. Archived from the original on June 9, 2010.
- ^ Goodwin, Bridget (1998). Keen as mustard: Britain's horrific chemical warfare experiments in Australia. St. Lucia: University of Queensland Press. ISBN 978-0-7022-2941-1.
- ^ Brook Island Trials of Mustard Gas during WW2. Home.st.net.au. Retrieved on 2011-05-29.
- ^ Dickerson, Caitlin (2015-06-23). "The VA's Broken Promise To Thousands Of Vets Exposed To Mustard Gas". NPR. Retrieved 2019-05-03.
... the Department of Veterans Affairs made two promises: to locate about 4,000 men who were used in the most extreme tests, and to compensate those who had permanent injuries.
- ^ Dickerson, Caitlin (2015-06-22). "Secret World War II Chemical Experiments Tested Troops By Race". NPR. Retrieved 2019-05-03.
And it wasn't just African-Americans. Japanese-Americans were used [...] so scientists could explore how mustard gas and other chemicals might affect Japanese troops. Puerto Rican soldiers were also singled out.
- ^ R. Baselt, Disposition of Toxic Drugs and Chemicals in Man, 10th edition, Biomedical Publications, Seal Beach, CA, 2014, pp. 1892–1894.
External links[]
| Wikimedia Commons has media related to Sulfur mustard. |
- Textbook of Military Medicine – Intensive overview of mustard gas Includes many references to scientific literature
- Detailed information on physical effects and suggested treatments
- Iyriboz Y (2004). "A Recent Exposure to Mustard Gas in the United States: Clinical Findings of a Cohort (n = 247) 6 Years After Exposure". MedGenMed. 6 (4): 4. PMC 1480580. PMID 15775831. Shows photographs taken in 1996 showing people with mustard gas burns.
- An overview of the sulfur and nitrogen mustard agents (Caution: contains graphic images)
- Questions and Answers for Mustard Gas
- UMDNJ-Rutgers University CounterACT Research Center of Excellence A research center studying mustard gas, includes searchable reference library with many early references on mustard gas.
- Clayton, William; Howard, Alfred John; Thomson, David (25 May 1946). "Treatment of Mustard Gas Burns". British Medical Journal. 1 (4455): 797–799. doi:10.1136/bmj.1.4455.797. PMC 2058956. PMID 20786722.
- Nightmare in Bari
- surgical treatment of mustard gas burns
- UK Ministry of Defence Report on disposal of weapons at sea and incidents arising
- Rhydymwyn Valley History Society
- The advent of mustard gas in 1917, Simon Jones
- Measures to protect against mustard gas, 1917-1918, Simon Jones
- Thioethers
- Organochlorides
- Blister agents
- World War I chemical weapons
- IARC Group 1 carcinogens
- Chloroethyl compounds
- Mustard compounds