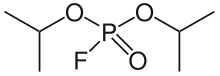
Diisopropyl fluorophosphate
 | |
 | |
| Clinical data | |
|---|---|
| Other names | isofluorophate, isofluorphate, DFP, DIFP, DIPF, diisopropyl phosphorofluoridate, EA-1152, PF-3, PF3, T-1703, TL 466 |
| ATC code | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.225 |
| Chemical and physical data | |
| Formula | C6H14FO3P |
| Molar mass | 184.147 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | −82 °C (−116 °F) |
| Boiling point | 183 °C (361 °F) 1013 mbar |
Diisopropyl fluorophosphate (DFP) or Isoflurophate is an oily, colorless liquid with the chemical formula C6H14FO3P. It is used in medicine[1] and as an organophosphorus insecticide[citation needed]. It is stable, but undergoes hydrolysis when subjected to moisture.
Uses in medicine[]
Diisopropyl fluorophosphate is a parasympathomimetic drug irreversible anti-cholinesterase and has been used in ophthalmology as a miotic agent in treatment of chronic glaucoma, as a miotic in veterinary medicine, and as an experimental agent in neuroscience because of its acetylcholinesterase inhibitory properties and ability to induce delayed peripheral neuropathy.[1]
Uses as toxin[]

The marked toxicity of esters of monofluorophosphoric acid was discovered in 1932, when and his PhD student Gerda von Krueger prepared the methyl, ethyl, n-propyl, and n-butyl esters and incidentally experienced their toxic effects. Another homologue of this series of esters, Diisopropyl fluorophosphate, was developed by British scientist . On his search for compounds to be used as chemical warfare agents, Saunders was inspired by the report by Lange and Krueger and decided to prepare the new homologue which he labeled PF-3. It was much less effective as a chemical weapon than the G series agents. It was often mixed with mustard gas, forming a more effective mixture with significantly lower melting point, resulting in an agent suitable for use in cold weather.

In military research, due to its physical and chemical similarities and comparatively low toxicity, it is used as a simulant of G-agents (GA, GB, GD, and GF). Diisopropyl fluorophosphate is used in civilian laboratories to mimic lethal nerve gas exposure or organophosphate toxicities.[2][3][4] It has also been used to develop a rodent model of Gulf War Syndrome.[5]
Diisopropyl fluorophosphate is a very potent neurotoxin. Its LD50 in rats is 6 mg/kg (oral). It combines with the amino acid serine at the active site of the enzyme acetylcholinesterase,[6] an enzyme that deactivates the neurotransmitter acetylcholine. Neurotransmitters are needed to continue the passage of nerve impulses from one neuron to another across the synapse. Once the impulse has been transmitted, acetylcholinesterase functions to deactivate the acetylcholine almost immediately by breaking it down. If the enzyme is inhibited, acetylcholine accumulates and nerve impulses cannot be stopped, causing prolonged muscle contraction. Paralysis occurs and death may result since the respiratory muscles are affected.
DFP also inhibits some proteases. It is a useful additive for protein or cell isolation procedure.
Production[]
Isoflurophate, the diisopropyl ester of fluorophosphoric acid, is made by reacting isopropyl alcohol with phosphorus trichloride, forming diisopropylphosphite, which is chlorinated and further reacted with sodium fluoride to replace the chlorine atom with fluorine, thus giving diisopropyl fluorophosphate.[7]
Biochemistry[]
DIPF is a diagnostic test for the presence of the active site Ser in serine proteases. The toxin, along with other neurotoxins is inactivated by the enzyme paraoxonase, which is present in widely varying levels in humans.
Society and culture[]
It is marketed under many brand names including Difluorophate, Diflupyl, Diflurphate, Dyflos, Dyphlos, Fluropryl, Fluostigmine, Neoglaucit.[citation needed]
See also[]
- MAFP (methoxy arachidonoylfluorophosphonate), a mechanistically related inhibitor
- Neopentylene fluorophosphate, a cyclic analogue
- Sarin (isopropyl methylphosphonofluridate), a related phosphofluridate
References[]
- ^ Jump up to: a b "Isofluorphate definition". Drugs.com. Archived from the original on 6 September 2017. Retrieved 6 September 2017.
- ^ Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ (August 2010). "Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate". Toxicological Sciences. 116 (2): 623–31. doi:10.1093/toxsci/kfq157. PMC 2905411. PMID 20498005.
- ^ Pessah IN, Rogawski MA, Tancredi DJ, Wulff H, Zolkowska D, Bruun DA, et al. (August 2016). "Models to identify treatments for the acute and persistent effects of seizure-inducing chemical threat agents". Annals of the New York Academy of Sciences. 1378 (1): 124–136. doi:10.1111/nyas.13137. PMC 5063690. PMID 27467073.
- ^ Kadriu B, Guidotti A, Costa E, Davis JM, Auta J (March 2011). "Acute imidazenil treatment after the onset of DFP-induced seizure is more effective and longer lasting than midazolam at preventing seizure activity and brain neuropathology". Toxicological Sciences. 120 (1): 136–45. doi:10.1093/toxsci/kfq356. PMID 21097996.
- ^ Phillips KF, Deshpande LS (January 2016). "Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness" (PDF). Neurotoxicology. 52: 127–33. doi:10.1016/j.neuro.2015.11.014. PMID 26619911.
- ^ Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, et al. (June 1999). "Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level". Biochemistry. 38 (22): 7032–9. doi:10.1021/bi982678l. PMID 10353814.
- ^ US patent 2409039, Hardy EE, Kosoloapoff GM, "Halogenated compounds and process for making same", issued 1946-10-08, assigned to Monsanto Chemical Company
Further reading[]
- Brenner GM (2000). Pharmacology. Philadelphia, PA: W.B. Saunders Company. ISBN 0-7216-7757-6.
- Meiers P (2006). "History of the fluorophosphates".
External links[]
- Organophosphate insecticides
- Acetylcholinesterase inhibitors
- Phosphorofluoridates
- Ophthalmology drugs
- Neurotoxins
- Isopropyl esters