From Wikipedia, the free encyclopedia
Kobophenol A
Names
Preferred IUPAC name
(2S ,2′R ,3S ,3′R )-3′-(3,5-Dihydroxyphenyl)-4-[(2S ,3S ,4R ,5S )-4-(3,5-dihydroxyphenyl)-2,5-bis(4-hydroxyphenyl)oxolan-3-yl]-2,2′-bis(4-hydroxyphenyl)-2,2′,3,3′-tetrahydro[3,4′-bi-1-benzofuran]-6,6′-diol
Other names
kob A
Identifiers
3D model (JSmol )
ChemSpider
InChI=1S/C56H44O13/c57-33-9-1-27(2-10-33)53-47(31-17-37(61)21-38(62)18-31)49-43(23-41(65)25-45(49)67-53)52-50-44(24-42(66)26-46(50)68-55(52)29-5-13-35(59)14-6-29)51-48(32-19-39(63)22-40(64)20-32)54(28-3-11-34(58)12-4-28)69-56(51)30-7-15-36(60)16-8-30/h1-26,47-48,51-66H/t47-,48+,51-,52+,53+,54-,55-,56-/m1/s1
N Key: RAUCCLKIJHMTND-LUPMIFTGSA-N
N InChI=1/C56H44O13/c57-33-9-1-27(2-10-33)53-47(31-17-37(61)21-38(62)18-31)49-43(23-41(65)25-45(49)67-53)52-50-44(24-42(66)26-46(50)68-55(52)29-5-13-35(59)14-6-29)51-48(32-19-39(63)22-40(64)20-32)54(28-3-11-34(58)12-4-28)69-56(51)30-7-15-36(60)16-8-30/h1-26,47-48,51-66H/t47-,48+,51-,52+,53+,54-,55-,56-/m1/s1
Key: RAUCCLKIJHMTND-LUPMIFTGBD
Oc1ccc(cc1)[C@@H]4Oc2cc(O)cc(c2[C@H]4c3cc(O)cc(O)c3)[C@H]6c7c(O[C@@H]6c5ccc(O)cc5)cc(O)cc7[C@H]%10[C@H](O[C@H](c8ccc(O)cc8)[C@H]%10c9cc(O)cc(O)c9)c%11ccc(O)cc%11
Properties
Chemical formula
C56 H44 O13
Molar mass
924.94 g/mol
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
N what is Y N
Infobox references
Chemical compound
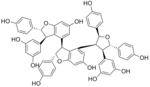
Kobophenol A is a stilbenoid. It is a tetramer of resveratrol . It can be isolated from Caragana chamlagu [1] Caragana sinica [2] [3]
The molecule shows a 2,3,4,5-tetraaryltetrahydrofuran skeleton.[3]
It has been shown to inhibit acetylcholinesterase .[1]
Acid-catalyzed epimerization of kobophenol A to carasinol B can be performed in vitro.[4]
References [ ]
^ a b (+)-α-Viniferin, a Stilbene Trimer from Caragana chamlague, Inhibits Acetylcholinesterase. Sang Hyun Sung, So Young Kang, Ki Yong Lee, Mi Jung Park, Jeong Hun Kim, Jong Hee Park, Young Chul Kim, Jinwoong Kim and Young Choong Kim, Biological & Pharmaceutical Bulletin, Vol. 25, 2002 [permanent dead link ^ Simultaneous determination of the contents of three stilbene oligomers in Caragana sinica collected in different seasons using an improved HPLC method. Shu Na; Zhou Hong; Hu Changqi, Biological & pharmaceutical bulletin, 2006, vol. 29, no4, pp. 608-612 ^ a b Identification and bioactivities of resveratrol oligomers and flavonoids from Carex folliculata seeds. Li L, Henry GE and Seeram NP, J Agric Food Chem., 26 August 2009, volume 57, issue 16, pages 7282-7287, doi :10.1021/jf901716j
^ Acid-catalyzed Epimerization of Kobophenol A to Carasinol B. Kejun Cheng, Gaolin Liang and Changqi Hu, Molecules 2008, 13(4), 938-942
Enzyme (modulators )
Transporter (modulators )
Release (modulators )
Inhibitors
SNAP-25 Botulinum toxin (A , , )VAMP Botulinum toxin (, , , ) Enhancers
LPHN α-Latrotoxin
See also: Receptor/signaling modulators • Muscarinic acetylcholine receptor modulators • Nicotinic acetylcholine receptor modulators
Dimers Trimers Tetramers:
(3"-hydroxygnetin E)
(3"-methoxygnetin E)
(isorhapontigenin tetramer)
and Higher polymers Oligomeric forms
Dimers Trimers Tetramers Pentamers Hexamers Higher polymers
Glycosides or conjugates
Diptoindonesin A (C-glucoside of ε-viniferin)Foeniculoside I (glucoside of miyabenol C), , and (an ε-viniferin-ascorbic acid hybrid compound)
(O-glucoside of ampelopsin A)
Categories :
Resveratrol oligomers Natural phenol tetramers Acetylcholinesterase inhibitors Hidden categories:
All articles with dead external links Articles with dead external links from February 2020 Articles with permanently dead external links Articles without EBI source Articles without KEGG source Articles without UNII source Chembox CAS registry number linked Articles with changed CASNo identifier Articles with changed ChemSpider identifier Articles with changed InChI identifier Pages using collapsible list with both background and text-align in titlestyle Articles containing unverified chemical infoboxes Chembox image size set Articles with short description Short description matches Wikidata