This article
needs additional citations for verification .
Please help by adding citations to reliable sources . Unsourced material may be challenged and removed.Find sources: – · · · scholar · JSTOR (September 2014 ) (Learn how and when to remove this template message )
Guanabenz AHFS /Drugs.com Consumer Drug Information MedlinePlus a686003 ATC code Protein binding 90% Elimination half-life 6 hours
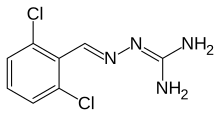
2-(2,6-dichlorobenzylidene)hydrazinecarboximidamide
CAS Number PubChem CID IUPHAR/BPS DrugBank ChemSpider UNII KEGG ChEMBL CompTox Dashboard (EPA ) ECHA InfoCard 100.023.410 Formula C 8 H 8 Cl 2 N 4 Molar mass −1 3D model (JSmol )
Clc1cccc(Cl)c1\C=N\N=C(/N)N
InChI=1S/C8H8Cl2N4/c9-6-2-1-3-7(10)5(6)4-13-14-8(11)12/h1-4H,(H4,11,12,14)/b13-4+
Y Key:WDZVGELJXXEGPV-YIXHJXPBSA-N
Y
Guanabenz (pronounced GWAHN-a-benz, sold under the trade name Wytensin ) is an alpha agonist of the alpha-2 adrenergic receptor that is used as an antihypertensive drug. It is used to treat high blood pressure (hypertension ).[1] [2]
The most common side effects during guanabenz therapy are dizziness , drowsiness , dry mouth , headache and weakness .
Guanabenz can make one drowsy or less alert, therefore driving or operating dangerous machinery is not recommended.
See also [ ] References [ ]
Sympatholytics α-adrenergic vasoconstriction )
Central
Peripheral
Indirect
Monoamine oxidase inhibitors VMAT inhibitors
Bietaserpine Deserpidine Methoserpidine Reserpine Syrosingopine Tyrosine hydroxylase inhibitors
Direct
Other antagonists
# WHO-EM ‡ Withdrawn from marketClinical trials :
† Phase III § Never to phase III
α1
Agonists Antagonists
Abanoquil Ajmalicine Alfuzosin Anisodamine Anisodine Atiprosin Atypical antipsychotics (e.g., brexpiprazole , clozapine , olanzapine , quetiapine , risperidone )Benoxathian Beta blockers (e.g., adimolol , amosulalol , arotinolol , carvedilol , eugenodilol , labetalol )Buflomedil Bunazosin Corynanthine Dapiprazole Domesticine Doxazosin Ergolines (e.g., acetergamine , ergotamine , dihydroergotamine , lisuride , nicergoline , terguride )Etoperidone Fenspiride Hydroxyzine Indoramin Ketanserin L-765,314 mCPP Mepiprazole Metazosin Monatepil Moxisylyte Naftopidil Nantenine Neldazosin Niaprazine Niguldipine Pardoprunox Pelanserin Perlapine Phendioxan Phenoxybenzamine Phentolamine Phenylpiperazine antidepressants (e.g., hydroxynefazodone , nefazodone , trazodone , triazoledione )Piperoxan Prazosin Quinazosin Quinidine Silodosin Spegatrine Spiperone Talipexole Tamsulosin Terazosin Tiodazosin Tolazoline Tetracyclic antidepressants (e.g., amoxapine , maprotiline , mianserin )Tricyclic antidepressants (e.g., amitriptyline , clomipramine , doxepin , imipramine , trimipramine )Trimazosin Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Urapidil WB-4101 Zolertine
α2
Agonists Antagonists
1-PP Adimolol Amesergide Aptazapine Atipamezole Atypical antipsychotics (e.g., asenapine , brexpiprazole , clozapine , lurasidone , olanzapine , paliperidone , quetiapine , risperidone , zotepine )Azapirones (e.g., buspirone , gepirone , ipsapirone , tandospirone )BRL-44408 Buflomedil Cirazoline Efaroxan Esmirtazapine Fenmetozole Fluparoxan Idazoxan Ketanserin Lisuride mCPP Mianserin Mirtazapine NAN-190 Pardoprunox Phentolamine Phenoxybenzamine Piperoxan Piribedil Rauwolscine Rotigotine Setiptiline Spegatrine Spiroxatrine Sunepitron Terguride Tolazoline Typical antipsychotics (e.g., chlorpromazine , fluphenazine , loxapine , thioridazine )Yohimbine
β
See also: Receptor/signaling modulators Dopaminergics Serotonergics Monoamine reuptake inhibitors Monoamine releasing agents Monoamine metabolism modulators Monoamine neurotoxins