From Wikipedia, the free encyclopedia
TRIO Available structures PDB Ortholog search: PDBe RCSB
Identifiers Aliases TRIO External IDs OMIM : 601893 MGI : 1927230 HomoloGene : 20847 GeneCards : TRIO Gene location (Human ) Chr. Chromosome 5 (human) [1] Band 5p15.2 Start 14,143,342 bp [1] End 14,532,128 bp [1]
Gene location (Mouse ) Chr. Chromosome 15 (mouse)[2] Band 15|15 B1 Start 27,730,651 bp [2] End 28,025,848 bp [2]
Gene ontology Molecular function Cellular component Biological process Sources:Amigo / QuickGO
Orthologs Species Human Mouse Entrez Ensembl UniProt RefSeq (mRNA) RefSeq (protein) Location (UCSC) Chr 5: 14.14 – 14.53 Mb Chr 15: 27.73 – 28.03 Mb PubMed search[3] [4] Wikidata
Triple functional domain protein is a protein that in humans is encoded by the TRIO gene .[5] [6]
Interactions [ ] TRIO (gene) has been shown to interact with Filamin [7] RHOA .[8]
References [ ]
^ a b c GRCh38: Ensembl release 89: ENSG00000038382 - Ensembl , May 2017^ a b c GRCm38: Ensembl release 89: ENSMUSG00000022263 - Ensembl , May 2017^ "Human PubMed Reference:" . National Center for Biotechnology Information, U.S. National Library of Medicine .^ "Mouse PubMed Reference:" . National Center for Biotechnology Information, U.S. National Library of Medicine .^ Debant A, Serra-Pagès C, Seipel K, O'Brien S, Tang M, Park SH, Streuli M (May 1996). "The multidomain protein Trio binds the LAR transmembrane tyrosine phosphatase, contains a protein kinase domain, and has separate rac-specific and rho-specific guanine nucleotide exchange factor domains" . Proceedings of the National Academy of Sciences of the United States of America . 93 (11): 5466–71. doi :10.1073/pnas.93.11.5466 PMC 39269 PMID 8643598 . ^ "Entrez Gene: TRIO triple functional domain (PTPRF interacting)" .^ Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A (Dec 2000). "The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin". Nature Cell Biology . 2 (12): 888–92. doi :10.1038/35046533 . PMID 11146652 . S2CID 10182923 . ^ Medley QG, Serra-Pagès C, Iannotti E, Seipel K, Tang M, O'Brien SP, Streuli M (Nov 2000). "The trio guanine nucleotide exchange factor is a RhoA target. Binding of RhoA to the trio immunoglobulin-like domain" . The Journal of Biological Chemistry . 275 (46): 36116–23. doi :10.1074/jbc.M003775200 PMID 10948190 .
Further reading [ ]
Taviaux S, Diriong S, Bellanger JM, Streuli M, Debant A (1997). "Assignment of TRIO, the Trio gene (PTPRF interacting) to human chromosome bands 5p 15.1-->p 14 by in situ hybridization". Cytogenetics and Cell Genetics . 76 (1–2): 107–8. doi :10.1159/000134524 . PMID 9154137 . Liu X, Wang H, Eberstadt M, Schnuchel A, Olejniczak ET, Meadows RP, Schkeryantz JM, Janowick DA, Harlan JE, Harris EA, Staunton DE, Fesik SW (Oct 1998). "NMR structure and mutagenesis of the N-terminal Dbl homology domain of the nucleotide exchange factor Trio" . Cell . 95 (2): 269–77. doi :10.1016/S0092-8674(00)81757-2 PMID 9790533 . S2CID 18146575 . Seipel K, Medley QG, Kedersha NL, Zhang XA, O'Brien SP, Serra-Pages C, Hemler ME, Streuli M (Jun 1999). "Trio amino-terminal guanine nucleotide exchange factor domain expression promotes actin cytoskeleton reorganization, cell migration and anchorage-independent cell growth". Journal of Cell Science . 112 ( Pt 12) (12): 1825–34. doi :10.1242/jcs.112.12.1825 . PMID 10341202 . Medley QG, Serra-Pagès C, Iannotti E, Seipel K, Tang M, O'Brien SP, Streuli M (Nov 2000). "The trio guanine nucleotide exchange factor is a RhoA target. Binding of RhoA to the trio immunoglobulin-like domain" . The Journal of Biological Chemistry . 275 (46): 36116–23. doi :10.1074/jbc.M003775200 PMID 10948190 . Bellanger JM, Astier C, Sardet C, Ohta Y, Stossel TP, Debant A (Dec 2000). "The Rac1- and RhoG-specific GEF domain of Trio targets filamin to remodel cytoskeletal actin". Nature Cell Biology . 2 (12): 888–92. doi :10.1038/35046533 . PMID 11146652 . S2CID 10182923 . Gao Y, Xing J, Streuli M, Leto TL, Zheng Y (Dec 2001). "Trp(56) of rac1 specifies interaction with a subset of guanine nucleotide exchange factors" . The Journal of Biological Chemistry . 276 (50): 47530–41. doi :10.1074/jbc.M108865200 PMID 11595749 . Skowronek KR, Guo F, Zheng Y, Nassar N (Sep 2004). "The C-terminal basic tail of RhoG assists the guanine nucleotide exchange factor trio in binding to phospholipids" . The Journal of Biological Chemistry . 279 (36): 37895–907. doi :10.1074/jbc.M312677200 PMID 15199069 . Zheng M, Simon R, Mirlacher M, Maurer R, Gasser T, Forster T, Diener PA, Mihatsch MJ, Sauter G, Schraml P (Jul 2004). "TRIO amplification and abundant mRNA expression is associated with invasive tumor growth and rapid tumor cell proliferation in urinary bladder cancer" . The American Journal of Pathology . 165 (1): 63–9. doi :10.1016/S0002-9440(10)63275-0 . PMC 1618551 PMID 15215162 . Yoshizuka N, Moriuchi R, Mori T, Yamada K, Hasegawa S, Maeda T, Shimada T, Yamada Y, Kamihira S, Tomonaga M, Katamine S (Oct 2004). "An alternative transcript derived from the trio locus encodes a guanosine nucleotide exchange factor with mouse cell-transforming potential" . The Journal of Biological Chemistry . 279 (42): 43998–4004. doi :10.1074/jbc.M406082200 PMID 15308664 . Portales-Casamar E, Briançon-Marjollet A, Fromont S, Triboulet R, Debant A (Mar 2006). "Identification of novel neuronal isoforms of the Rho-GEF Trio". Biology of the Cell . 98 (3): 183–93. doi :10.1042/BC20050009 . PMID 16033331 . S2CID 24166861 . Tao WA, Wollscheid B, O'Brien R, Eng JK, Li XJ, Bodenmiller B, Watts JD, Hood L, Aebersold R (Aug 2005). "Quantitative phosphoproteome analysis using a dendrimer conjugation chemistry and tandem mass spectrometry". Nature Methods . 2 (8): 591–8. doi :10.1038/nmeth776 . PMID 16094384 . S2CID 20475874 . Adamowicz M, Radlwimmer B, Rieker RJ, Mertens D, Schwarzbach M, Schraml P, Benner A, Lichter P, Mechtersheimer G, Joos S (Sep 2006). "Frequent amplifications and abundant expression of TRIO, NKD2, and IRX2 in soft tissue sarcomas". Genes, Chromosomes & Cancer . 45 (9): 829–38. doi :10.1002/gcc.20343 . PMID 16752383 . S2CID 24491021 . Olsen JV, Blagoev B, Gnad F, Macek B, Kumar C, Mortensen P, Mann M (Nov 2006). "Global, in vivo, and site-specific phosphorylation dynamics in signaling networks" . Cell . 127 (3): 635–48. doi :10.1016/j.cell.2006.09.026 PMID 17081983 . S2CID 7827573 . Chhatriwala MK, Betts L, Worthylake DK, Sondek J (May 2007). "The DH and PH domains of Trio coordinately engage Rho GTPases for their efficient activation" . Journal of Molecular Biology . 368 (5): 1307–20. doi :10.1016/j.jmb.2007.02.060 . PMC 1890047 PMID 17391702 . Rojas RJ, Yohe ME, Gershburg S, Kawano T, Kozasa T, Sondek J (Oct 2007). "Galphaq directly activates p63RhoGEF and Trio via a conserved extension of the Dbl homology-associated pleckstrin homology domain" . The Journal of Biological Chemistry . 282 (40): 29201–10. doi :10.1074/jbc.M703458200 PMC 2655113 PMID 17606614 .
External links [ ] Overview of all the structural information available in the PDB for UniProt : O75962 PDBe-KB .
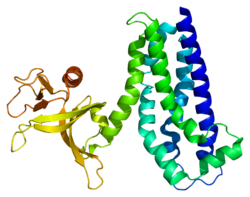
PDB gallery
1nty : Crystal structure of the first DH/PH domain of Trio to 1.7 A
2nz8 : N-terminal DHPH cassette of Trio in complex with nucleotide-free Rac1
Enzymes
Activity Regulation Classification
EC number Enzyme superfamily Enzyme family List of enzymes Kinetics Types
Categories :
Genes on human chromosome 5 EC 2.7.11 Human chromosome 5 gene stubs Hidden categories:
CS1: long volume value All stub articles