Testicle
| Testicle | |
|---|---|
 Diagram of inner structures of testicles | |
 Diagram of the external features and surrounding structures of the testicles of an adult male | |
| Details | |
| Artery | Testicular artery |
| Vein | Testicular vein, Pampiniform plexus |
| Nerve | Spermatic plexus |
| Lymph | Lumbar lymph nodes |
| Identifiers | |
| Latin | testis |
| MeSH | D013737 |
| TA98 | A09.3.01.001 |
| TA2 | 3576 |
| FMA | 7210 |
| Anatomical terminology | |
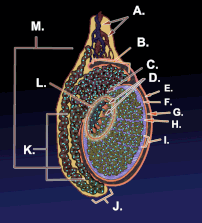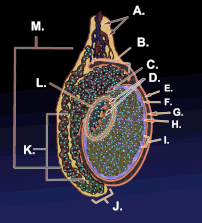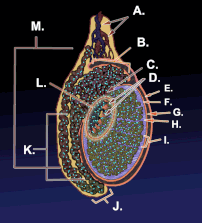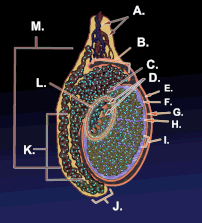
Testicle or testis (plural testes) is the male reproductive gland or gonad in all animals, including humans. It is homologous to the female ovary. The functions of the testes are to produce both sperm and androgens, primarily testosterone. Testosterone release is controlled by the anterior pituitary luteinizing hormone, whereas sperm production is controlled both by the anterior pituitary follicle-stimulating hormone and gonadal testosterone.
Structure
Appearance

Males have two testicles of similar size contained within the scrotum, which is an extension of the abdominal wall. Scrotal asymmetry is not unusual: one testicle extends farther down into the scrotum than the other due to differences in the anatomy of the vasculature.
Measurement
The volume of the testicle can be estimated by palpating it and comparing it to ellipsoids of known sizes. Another method is to use calipers (an orchidometer) or a ruler either on the person or on an ultrasound image to obtain the three measurements of the x, y, and z axes (length, depth and width). These measurements can then be used to calculate the volume, using the formula for the volume of an ellipsoid:
An average adult testicle measures up to 5 cm × 2 cm × 3 cm (2 in × 3⁄4 in × 1+1⁄4 in). The Tanner scale for the maturity of male genitals assigns a maturity stage to the calculated volume ranging from stage I, a volume of less than 1.5 cm3; to stage V, a volume greater than 20 cm3. Normal volume is 15 to 25 cm3; the average is 18 cm3 per testis (range 12–30 cm3).[1]
Internal structure
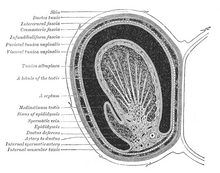
Duct system
The testes are covered by a tough membranous shell called the tunica albuginea. Within the testes are very fine coiled tubes called seminiferous tubules. The tubules are lined with a layer of cells (germ cells) that develop from puberty through old age into sperm cells (also known as spermatozoa or male gametes). The developing sperm travel through the seminiferous tubules to the rete testis located in the mediastinum testis, to the efferent ducts, and then to the epididymis where newly created sperm cells mature (see spermatogenesis). The sperm move into the vas deferens, and are eventually expelled through the urethra and out of the urethral orifice through muscular contractions.
Primary cell types
Within the seminiferous tubules, the germ cells develop into spermatogonia, spermatocytes, spermatids and spermatozoon through the process of spermatogenesis. The gametes contain DNA for fertilization of an ovum[2] Sertoli cells – the true epithelium of the seminiferous epithelium, critical for the support of germ cell development into spermatozoa. Sertoli cells secrete inhibin.[3] Peritubular myoid cells surround the seminiferous tubules.[4]
Between tubules (interstitial cells), exist Leydig cells – cells localized between seminiferous tubules that produce and secrete testosterone and other androgens important for sexual development and puberty, secondary sexual characteristics like facial hair, sexual behavior and libido. They also support spermatogenesis and erectile function. Testosterone controls testicular volume.
Immature Leydig cells and interstitial macrophages and epithelial cells are also present.
Blood supply and lymphatic drainage
Blood supply and lymphatic drainage of the testes and scrotum are distinct:
- The paired testicular arteries arise directly from the abdominal aorta and descend through the inguinal canal, while the scrotum and the rest of the external genitalia is supplied by the internal pudendal artery (itself a branch of the internal iliac artery).
- The testis has collateral blood supply from 1. the cremasteric artery (a branch of the inferior epigastric artery, which is a branch of the external iliac artery), and 2. the artery to the ductus deferens (a branch of the inferior vesical artery, which is a branch of the internal iliac artery). Therefore, if the testicular artery is ligated, e.g., during a Fowler-Stevens orchiopexy for a high undescended testis, the testis will usually survive on these other blood supplies.
- Lymphatic drainage of the testes follows the testicular arteries back to the paraaortic lymph nodes, while lymph from the scrotum drains to the inguinal lymph nodes.
Layers
Many anatomical features of the adult testis reflect its developmental origin in the abdomen. The layers of tissue enclosing each testicle are derived from the layers of the anterior abdominal wall. Notably, the cremasteric muscle arises from the internal oblique muscle.
The blood–testis barrier
Large molecules cannot pass from the blood into the lumen of a seminiferous tubule due to the presence of tight junctions between adjacent Sertoli cells. The spermatogonia are in the basal compartment (deep to the level of the tight junctions) and the more mature forms such as primary and secondary spermatocytes and spermatids are in the adluminal compartment.
The function of the blood–testis barrier may be to prevent an auto-immune reaction. Mature sperm (and their antigens) arise long after immune tolerance is established in infancy. Since sperm are antigenically different from self tissue, a male animal can react immunologically to his own sperm. He is capable of making antibodies against them.
Injection of sperm antigens causes inflammation of the testis (auto-immune orchitis) and reduced fertility. The blood–testis barrier may reduce the likelihood that sperm proteins will induce an immune response, reducing fertility and so progeny.
Temperature regulation
Spermatogenesis is enhanced at temperatures slightly less than core body temperature.[5] The spermatogenesis is less efficient at lower and higher temperatures than 33 °C.[5] Because the testes are located outside the body, the smooth tissue of the scrotum can move them closer or further away from the body.[5] The temperature of the testes is maintained at 35 degrees Celsius (95 degrees Fahrenheit), i.e. two degrees below the body temperature of 37 degrees Celsius (98.6 degrees Fahrenheit). Higher temperatures affect spermatogenesis.[6] There are a number of mechanisms to maintain the testes at the optimum temperature.[5]
The cremasteric muscle is part of the spermatic cord. When this muscle contracts, the cord is shortened and the testicle is moved closer up toward the body, which provides slightly more warmth to maintain optimal testicular temperature. When cooling is required, the cremasteric muscle relaxes and the testicle is lowered away from the warm body and is able to cool. Contraction also occurs in response to stress (the testicles rise up toward the body in an effort to protect them in a fight).
The cremaster muscle can reflexively raise each testicle individually if properly triggered. This phenomenon is known as the cremasteric reflex. The testicles can also be lifted voluntarily using the pubococcygeus muscle, which partially activates related muscles.
Gene and protein expression
The human genome includes approximately 20,000 protein coding genes: 80% of these genes are expressed in adult testes.[7] The testes have the highest fraction of tissue type-specific genes compared to other organs and tissues:[8] about 1000 of them are highly specific for the testes,[7] and about 2,200 show an elevated pattern of expression here. A majority of these genes encode for proteins that are expressed in the seminiferous tubules and have functions related to spermatogenesis.[9][8] Sperm cells express proteins that result in the development of flagella; these same proteins are expressed in the female in cells lining the fallopian tube, and cause the development of cilia. In other words, sperm cell flagella and Fallopian tube cilia are homologous structures. The testis-specific proteins that show the highest level of expression are protamines.
Development
There are two phases in which the testes grow substantially; namely in embryonic and pubertal age.
Embryonic
During mammalian development, the gonads are at first capable of becoming either ovaries or testes.[10] In humans, starting at about week 4 the gonadal rudiments are present within the intermediate mesoderm adjacent to the developing kidneys. At about week 6, sex cords develop within the forming testes. These are made up of early Sertoli cells that surround and nurture the germ cells that migrate into the gonads shortly before sex determination begins. In males, the sex-specific gene SRY that is found on the Y-chromosome initiates sex determination by downstream regulation of sex-determining factors, (such as GATA4, SOX9 and AMH), which leads to development of the male phenotype, including directing development of the early bipotential gonad down the male path of development.
Testes follow the "path of descent" from high in the posterior fetal abdomen to the inguinal ring and beyond to the inguinal canal and into the scrotum. In most cases (97% full-term, 70% preterm), both testes have descended by birth. In most other cases, only one testis fails to descend (cryptorchidism) and that will probably express itself within a year.
Pubertal
The testes grow in response to the start of spermatogenesis. Size depends on lytic function, sperm production (amount of spermatogenesis present in testis), interstitial fluid, and Sertoli cell fluid production. After puberty, the volume of the testes can be increased by over 500% as compared to the pre-pubertal size.[citation needed] Testicles are fully descended before one reaches puberty.
Clinical significance
Protection and injury
- The testicles are well known to be very sensitive to impact and injury. The pain involved travels up from each testicle into the abdominal cavity, via the spermatic plexus, which is the primary nerve of each testicle. This will cause pain in the hip and the back. The pain usually goes away in a few minutes.
- Testicular torsion is a medical emergency.
- Testicular rupture is a medical emergency caused by blunt force impact, sharp edge, or piercing impact to one or both testicles, which can lead to necrosis of the testis in as little as 30 minutes.[citation needed]
- Penetrating injuries to the scrotum may cause castration, or physical separation or destruction of the testes, possibly along with part or all of the penis, which results in total sterility if the testicles are not reattached quickly.
- Some jockstraps are designed to provide support to the testicles.[11]
Diseases and conditions
| Testicular disease | |
|---|---|
| Specialty | Urology, Reproductive medicine |
- Testicular cancer and other neoplasms – To improve the chances of catching possible cases of testicular cancer or other health issues early, regular testicular self-examination is recommended.
- Varicocele, swollen vein(s) from the testes, usually affecting the left side,[12] the testis usually being normal
- Hydrocele testis, swelling around testes caused by accumulation of clear liquid within a membranous sac, the testis usually being normal
- Spermatocele, a retention cyst of a tubule of the rete testis or the head of the epididymis distended with barely watery fluid that contains spermatozoa
- Endocrine disorders can also affect the size and function of the testis.
- Certain inherited conditions involving mutations in key developmental genes also impair testicular descent, resulting in abdominal or inguinal testes which remain nonfunctional and may become cancerous. Other genetic conditions can result in the loss of the Wolffian ducts and allow for the persistence of Müllerian ducts. Both excess and deficient levels of estrogens can disrupt spermatogenesis and cause infertility.[13]
- Bell-clapper deformity is a deformity in which the testicle is not attached to the scrotal walls, and can rotate freely on the spermatic cord within the tunica vaginalis. It is the most common underlying cause of testicular torsion.
- Orchitis inflammation of the testicles
- Epididymitis, a painful inflammation of the epididymis or epididymides frequently caused by bacterial infection but sometimes of unknown origin.
- Anorchia, the absence of one or both testicles.
- Cryptorchidism or "undescended testicles", when the testicle does not descend into the scrotum of the infant boy.
Testicular enlargement is an unspecific sign of various testicular diseases, and can be defined as a testicular size of more than 5 cm (long axis) × 3 cm (short axis).[14]
Blue balls is a slang term for a temporary fluid congestion in the testicles and prostate region caused by prolonged sexual arousal.
Testicular prostheses are available to mimic the appearance and feel of one or both testicles, when absent as from injury or as treatment in association to gender dysphoria. There have also been some instances of their implantation in dogs.[15]
Effects of exogenous hormones
To some extent, it is possible to change testicular size. Short of direct injury or subjecting them to adverse conditions, e.g., higher temperature than they are normally accustomed to, they can be shrunk by competing against their intrinsic hormonal function through the use of externally administered steroidal hormones. Steroids taken for muscle enhancement (especially anabolic steroids) often have the undesired side effect of testicular shrinkage.
Similarly, stimulation of testicular functions via gonadotropic-like hormones may enlarge their size. Testes may shrink or atrophy during hormone replacement therapy or through chemical castration.
In all cases, the loss in testes volume corresponds with a loss of spermatogenesis.
Society and culture
The testicles of calves, lambs, roosters, turkeys, and other animals are eaten in many parts of the world, often under euphemistic culinary names. Testicles are a by-product of the castration of young animals raised for meat, so they were probably a late-spring seasonal specialty,[16] though nowadays they are generally frozen and available year-round.
As early as 330 BC, Aristotle prescribed the ligation (tying off) of the left testicle in men wishing to have boys.[17] In the Middle Ages, men who wanted a boy sometimes had their left testicle removed. This was because people believed that the right testicle made "boy" sperm and the left made "girl" sperm.[18]
Etymology and slang
One theory about the etymology of the word testis is based on Roman law. The original Latin word testis, "witness", was used in the firmly established legal principle "Testis unus, testis nullus" (one witness [equals] no witness), meaning that testimony by any one person in court was to be disregarded unless corroborated by the testimony of at least another. This led to the common practice of producing two witnesses, bribed to testify the same way in cases of lawsuits with ulterior motives. Since such "witnesses" always came in pairs, the meaning was accordingly extended, often in the diminutive (testiculus, testiculi).[citation needed]
Another theory says that testis is influenced by a loan translation, from Greek parastatēs "defender (in law), supporter" that is "two glands side by side".[19]
In slang, the testes are usually referred to as "balls" as a reference to blue balls. Frequently, "nuts" (sometimes intentionally misspelled as "nutz") are also a slang term for the testes due to the geometric resemblance, as evidenced by the various usages of the term "Deez Nuts", which include a satirical political candidate in 2016.
Other animals
External appearance
In sharks, the testicle on the right side is usually larger, and in many bird and mammal species, the left may be the larger. The primitive jawless fish have only a single testis, located in the midline of the body, although even this forms from the fusion of paired structures in the embryo.[20]
In seasonal breeders, the weight of the testes often increases during the breeding season.[21] The testicles of a dromedary camel are 7–10 cm (2.8–3.9 in) long, 4.5 cm (1.8 in) deep and 5 cm (2.0 in) in width. The right testicle is often smaller than the left.[22]
Location
Internal
The basal condition for mammals is to have internal testes.[23] The testes of monotremes,[24][25] xenarthrans,[25] and elephants[26] remain within the abdomen. There are also some marsupials with external testes[27][28][29] and Boreoeutherian mammals with internal testes, such as the rhinoceros.[30] Cetaceans such as whales and dolphins also have internal testes.[31][32] As external testes would increase drag in the water they have internal testes which are kept cool by special circulatory systems that cool the arterial blood going to the testes by placing the arteries near veins bringing cooled venous blood from the skin.[33][34] In odobenids and phocids, the location of the testes is para-abdominal, though otariids have scrotal testes.[35]
External
Boreoeutherian land mammals, the large group of mammals that includes humans, have externalized testes.[36] Their testes function best at temperatures lower than their core body temperature. Their testes are located outside of the body, suspended by the spermatic cord within the scrotum.
There are several hypotheses why most boreotherian mammals have external testes which operate best at a temperature that is slightly less than the core body temperature, e.g. that it is stuck with enzymes evolved in a colder temperature due to external testes evolving for different reasons, that the lower temperature of the testes simply is more efficient for sperm production.
1) More efficient. The classic hypothesis is that cooler temperature of the testes allows for more efficient fertile spermatogenesis. In other words, there are no possible enzymes operating at normal core body temperature that are as efficient as the ones evolved, at least none appearing in our evolution so far.
The early mammals had lower body temperatures and thus their testes worked efficiently within their body. However it is argued that boreotherian mammals have higher body temperatures than the other mammals and had to develop external testes to keep them cool. It is argued that those mammals with internal testes, such as the monotremes, armadillos, sloths, elephants, and rhinoceroses, have a lower core body temperatures than those mammals with external testes.[citation needed]
However, the question remains why birds despite having very high core body temperatures have internal testes and did not evolve external testes.[37] It was once theorized that birds used their air sacs to cool the testes internally, but later studies revealed that birds' testes are able to function at core body temperature.[37]
Some mammals which have seasonal breeding cycles keep their testes internal until the breeding season at which point their testes descend and increase in size and become external.[38]
2) Irreversible adaptation to sperm competition. It has been suggested that the ancestor of the boreoeutherian mammals was a small mammal that required very large testes (perhaps rather like those of a hamster) for sperm competition and thus had to place its testes outside the body.[39] This led to enzymes involved in spermatogenesis, spermatogenic DNA polymerase beta and recombinase activities evolving a unique temperature optimum, slightly less than core body temperature. When the boreoeutherian mammals then diversified into forms that were larger and/or did not require intense sperm competition they still produced enzymes that operated best at cooler temperatures and had to keep their testes outside the body. This position is made less parsimonious by the fact that the kangaroo, a non-boreoeutherian mammal, has external testicles. The ancestors of kangaroos might, separately from boreotherian mammals, have also been subject to heavy sperm competition and thus developed external testes, however, kangaroo external testes are suggestive of a possible adaptive function for external testes in large animals.
3) Protection from abdominal cavity pressure changes. One argument for the evolution of external testes is that it protects the testes from abdominal cavity pressure changes caused by jumping and galloping.[40]
4) Protection against DNA damage. Mild, transient scrotal heat stress causes DNA damage, reduced fertility and abnormal embryonic development in mice.[41] DNA strand breaks were found in spermatocytes recovered from testicles subjected to 40 °C or 42 °C for 30 minutes.[41] These findings suggest that the external location of the testicles provides the adaptive benefit of protecting spermatogenic cells from heat-induced DNA damage that could otherwise lead to infertility and germline mutation.
Size

The relative size of testes is often influenced by mating systems.[42] Testicular size as a proportion of body weight varies widely. In the mammalian kingdom, there is a tendency for testicular size to correspond with multiple mates (e.g., harems, polygamy). Production of testicular output sperm and spermatic fluid is also larger in polygamous animals, possibly a spermatogenic competition for survival. The testes of the right whale are likely to be the largest of any animal, each weighing around 500 kg (1,100 lb).[43]
Among the Hominidae, gorillas have little female promiscuity and sperm competition and the testes are small compared to body weight (0.03%). Chimpanzees have high promiscuity and large testes compared to body weight (0.3%). Human testicular size falls between these extremes (0.08%).[44]
Testis weight also varies in seasonal breeders like red foxes,[45] golden jackals[46] and coyotes.[21]
Internal structure
Under a tough membranous shell called the tunica albuginea, the testis of amniotes, as well as some teleost fish, contains very fine coiled tubes called seminiferous tubules.
Amphibians and most fish do not possess seminiferous tubules. Instead, the sperm are produced in spherical structures called sperm ampullae. These are seasonal structures, releasing their contents during the breeding season, and then being reabsorbed by the body. Before the next breeding season, new sperm ampullae begin to form and ripen. The ampullae are otherwise essentially identical to the seminiferous tubules in higher vertebrates, including the same range of cell types.[20]
Gallery
Testicle
Testicle
Testicle hanging on cremaster muscle. These are two healthy testicles. Heat causes them to descend, allowing cooling.
A healthy scrotum containing normal size testes. The scrotum is in tight condition. The image also shows the texture.
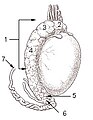
Testicle of a cat: 1: Extremitas capitata, 2: Extremitas caudata, 3: Margo epididymalis, 4: Margo liber, 5: Mesorchium, 6: Epididymis, 7: testicular artery and vene, 8: Ductus deferens
Testis surface

Testis cross section

The right testis, exposed by laying open the tunica vaginalis.

Microscopic view of Rabbit testis 100×
Testicle
See also
- Anorchia
- Bollocks
- Castration
- Cryptorchidism (cryptorchismus)
- Ejaculation
- Epididymis
- Eunuchs
- Gelding
- Infertility
- List of homologues of the human reproductive system
- Neutering
- Orchidometer
- Penis
- Perineum
- Polyorchidism
- Spermatic cord
- Spermatogenesis
- Sterilization (surgical procedure), vasectomy
- Testicles as food
- Testicondy
- Testicular nubbin
- Testosterone
Notes
- Heptner, V. G.; Naumov, N. P. (1998). Mammals of the Soviet Union Vol. II Part 1a, SIRENIA AND CARNIVORA (Sea cows; Wolves and Bears). Enfield, NH: Science Publishers. ISBN 978-1-886106-81-9. OCLC 490089621. Retrieved 9 November 2013.
References
- ^ Andrology: Male Reproductive Health and Dysfunction by E. Nieschlag, Hermann M. Behre, H. van. Ahlen
- ^ Histology, A Text and Atlas by Michael H. Ross and Wojciech Pawlina, Lippincott Williams & Wilkins, 5th ed, 2006
- ^ Skinner M, McLachlan R, Bremner W (1989). "Stimulation of Sertoli cell inhibin secretion by the testicular paracrine factor PModS". Mol Cell Endocrinol. 66 (2): 239–49. doi:10.1016/0303-7207(89)90036-1. hdl:1773/4395. PMID 2515083. S2CID 21885326.
- ^ Arch Histol Cytol. 1996 Mar;59(1):1–13
- ^ a b c d Mieusset, R; Bujan, L; Mansat, A; Pontonnier, F (1992). "Hyperthermie scrotale et infécondité masculine" (PDF). Progrès en Urologie (in French) (2): 31–36. Archived from the original (PDF) on 2016-03-03. Retrieved 2016-04-25.
- ^ 1942-, Van De Graaff, Kent M. (Kent Marshall) (1989). Concepts of human anatomy and physiology. Fox, Stuart Ira. (2nd ed.). Dubuque, Iowa: Wm. C. Brown Publishers. pp. 936. ISBN 978-0697056757. OCLC 19493880.CS1 maint: numeric names: authors list (link)
- ^ a b Uhlén, Mathias; Fagerberg, Linn; Hallström, Björn M.; Lindskog, Cecilia; Oksvold, Per; Mardinoglu, Adil; Sivertsson, Åsa; Kampf, Caroline; Sjöstedt, Evelina (2015-01-23). "Tissue-based map of the human proteome". Science. 347 (6220): 1260419. doi:10.1126/science.1260419. ISSN 0036-8075. PMID 25613900. S2CID 802377.
- ^ a b Djureinovic, D.; Fagerberg, L.; Hallström, B.; Danielsson, A.; Lindskog, C.; Uhlén, M.; Pontén, F. (2014-06-01). "The human testis-specific proteome defined by transcriptomics and antibody-based profiling". MHR: Basic Science of Reproductive Medicine. 20 (6): 476–488. doi:10.1093/molehr/gau018. ISSN 1360-9947. PMID 24598113.
- ^ "The human proteome in testis - The Human Protein Atlas". www.proteinatlas.org.
- ^ Online textbook: "Developmental Biology" 6th ed. By Scott F. Gilbert (2000) published by Sinauer Associates, Inc. of Sunderland (MA).
- ^ "Amazon.com: Suspensory Jockstrap for Scrotal/Testicle Support by FlexaMed: Health & Personal Care". www.Amazon.com. Retrieved 25 January 2018.
- ^ "Varicocele". Kidshealth.org. Retrieved 25 October 2010.
- ^ Sierens, J. E.; Sneddon, S. F.; Collins, F.; Millar, M. R.; Saunders, P. T. (2005). "Estrogens in Testis Biology". Annals of the New York Academy of Sciences. 1061 (1): 65–76. Bibcode:2005NYASA1061...65S. doi:10.1196/annals.1336.008. PMID 16467258. S2CID 24905596.
- ^ Page 559 in: John Pellerito, Joseph F Polak (2012). Introduction to Vascular Ultrasonography (6 ed.). Elsevier Health Sciences. ISBN 9781455737666.
- ^ "Neuticles.com". www.Neuticles.com. Retrieved 25 January 2018.
- ^ Mason, Laura (2014). Davidson, Alan (ed.). The Oxford Companion to Food. Oxford University Press. p. 816. ISBN 9780199677337.
- ^ Hoag, Hannah. I'll take a girl, please... Cherry-picking from the dish of life. Drexel University Publication. Archived August 31, 2011, at the Wayback Machine
- ^ "Understanding Genetics". Genetics.TheTech.org. Retrieved 25 January 2018.
- ^ The American Heritage Dictionary of the English Language, Fourth Edition
- ^ a b Romer, Alfred Sherwood; Parsons, Thomas S. (1977). The Vertebrate Body. Philadelphia, PA: Holt-Saunders International. pp. 385–386. ISBN 978-0-03-910284-5.
- ^ a b A.D. Johnson (2 December 2012). Development, Anatomy, and Physiology. Elsevier. ISBN 978-0-323-14323-3.
- ^ Mukasa-Mugerwa, E. The Camel (Camelus dromedarius): A Bibliographical Review. pp. 11–3.
- ^ "Archived copy". Archived from the original on 2018-02-25. Retrieved 2018-12-08.CS1 maint: archived copy as title (link)
- ^ Mervyn Griffiths (2 December 2012). The Biology of the Monotremes. Elsevier Science. ISBN 978-0-323-15331-7.
- ^ a b Ronald M. Nowak; Ernest Pillsbury Walker (29 July 1999). Walker's Mammals of the World. JHU Press. ISBN 978-0-8018-5789-8.
testes.
- ^ Murray Fowler; Susan K. Mikota (9 January 2008). Biology, Medicine, and Surgery of Elephants. John Wiley & Sons. ISBN 978-0-470-34411-8.
- ^ Don II Hunsaker (2 December 2012). The Biology of Marsupials. Elsevier Science. ISBN 978-0-323-14620-3.
- ^ C. Hugh Tyndale-Biscoe (2005). Life of Marsupials. Csiro Publishing. ISBN 978-0-643-06257-3.
- ^ Hugh Tyndale-Biscoe; Marilyn Renfree (30 January 1987). Reproductive Physiology of Marsupials. Cambridge University Press. ISBN 978-0-521-33792-2.
- ^ Schaffer, N. E., et al. "Ultrasonography of the reproductive anatomy in the Sumatran rhinoceros (Dicerorhinus sumatrensis)." Journal of Zoo and Wildlife Medicine (1994): 337-348.
- ^ Bernd Würsig; J.G.M. Thewissen; Kit M. Kovacs (27 November 2017). Encyclopedia of Marine Mammals. Elsevier Science. ISBN 978-0-12-804381-3.
- ^ Rommel, Sentiel A., D. Ann Pabst, and William A. McLellan. "Functional anatomy of the cetacean reproductive system, with comparisons to the domestic dog." Reproductive Biology and Phylogeny of Cetacea. Science Publishers (2016): 127–145.
- ^ Rommel, Sentiel A., D. Ann Pabst, and William A. McLellan. "Reproductive Thermoregulation in Marine Mammals: How do male cetaceans and seals keep their testes cool without a scrotum? It turns out to be the same mechanism that keeps the fetus cool in a pregnant female Archived 2017-09-23 at the Wayback Machine." American scientist 86.5 (1998): 440-448.
- ^ Pabst, D. Ann, Sentiel A. Rommel, and WILLIAM A. McLELLAN. "Evolution of thermoregulatory function in cetacean reproductive systems." The Emergence of Whales. Springer US, 1998. 379-397.
- ^ Frances M.D. Gulland; Leslie A. Dierauf; Karyl L. Whitman (20 March 2018). CRC Handbook of Marine Mammal Medicine. CRC Press. ISBN 978-1-351-38416-2.
- ^ D. S. Mills; Jeremy N. Marchant-Forde (2010). The Encyclopedia of Applied Animal Behaviour and Welfare. CABI. pp. 293–. ISBN 978-0-85199-724-7.
- ^ a b BIOLOGY OF REPRODUCTION 56, 1570–1575 (1997)- Determination of Testis Temperature Rhythms and Effects of Constant Light on Testicular Function in the Domestic Fowl (Gallus domesticus) Archived 2015-09-23 at the Wayback Machine
- ^ "Ask a Biologist Q&A / Human sexual physiology – good design?". Askabiologist.org.uk. 4 September 2007. Retrieved 25 October 2010.
- ^ "'The Human Body as an Evolutionary Patchwork' by Alan Walker, Princeton.edu". RichardDawkins.net. 2007-04-10. Archived from the original on 9 November 2013. Retrieved 25 October 2010.
- ^ "Science : Bumpy lifestyle led to external testes".
- ^ a b Paul C, Murray AA, Spears N, Saunders PT (2008). "A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice". Reproduction. 136 (1): 73–84. doi:10.1530/REP-08-0036. PMID 18390691.
- ^ Pitcher, T.E.; Dunn, P.O.; Whittingham, L.A. (2005). "Sperm competition and the evolution of testes size in birds". Journal of Evolutionary Biology. 18 (3): 557–567. doi:10.1111/j.1420-9101.2004.00874.x. PMID 15842485. S2CID 18331398.
- ^ Crane, J.; Scott, R. (2002). "Eubalaena glacialis". Animal Diversity Web. Retrieved 1 May 2009.
- ^ Shackelford, T. K.; Goetz, A. T. (2007). "Adaptation to Sperm Competition in Humans". Current Directions in Psychological Science. 16: 47–50. doi:10.1111/j.1467-8721.2007.00473.x. S2CID 6179167.
- ^ Heptner & Naumov 1998, p. 537
- ^ Heptner & Naumov 1998, pp. 154–155
External links
- Testicle
- Endocrine system
- Glands
- Mammal male reproductive system
- Men's health
- Scrotum












