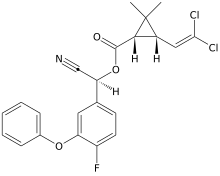
Cyfluthrin
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
(R)-Cyano(4-fluoro-3-phenoxyphenyl)methyl (1R,3R)-3-(2,2-dichloroethen-1-yl)-2,2-dimethylcyclopropane-1-carboxylate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.063.485 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C22H18Cl2FNO3 | |
| Molar mass | 434.29 g·mol−1 |
| Melting point | 60 °C (140 °F; 333 K) |
| 2 μg/L | |
| Pharmacology | |
| P03BA01 (WHO) QP53AC12 (WHO) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cyfluthrin is a pyrethroid insecticide and common household pesticide. It is a complex organic compound and the commercial product is sold as a mixture of isomers. Like most pyrethroids (MoA 3a),[1] it is highly toxic to fish and invertebrates, but it is far less toxic to humans.[2] It is generally supplied as a 10–25% liquid concentrate for commercial use and is diluted prior to spraying onto agricultural crops and outbuildings.
Safety[]
In rats, the LD50s are 500, 800 (oral), and 600 (skin) mg/kg.[2]
Excessive exposure can cause nausea, headache, muscle weakness, salivation, shortness of breath and seizures. In humans, it is deactivated by enzymatic hydrolysis to several carboxylic acid metabolites, whose urinary excretion half-lives are in a range of 5–7 hours. Worker exposure to the chemical can be monitored by measurement of the urinary metabolites, while severe overdosage may be confirmed by quantification of cyfluthrin in blood or plasma.[3]
Health and safety risks are controlled by right to know laws that exist in most developed countries. Cyfluthrin is regulated in the US by the EPA.[4]
See also[]
References[]
- ^ "IRAC Mode of Action Classification Scheme Version 9.4". IRAC (Insecticide Resistance Action Committee) (pdf). March 2020.
- ^ a b Robert L. Metcalf (2002). "Insect Control". Ullmann’s Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a14_263.
- ^ R. Baselt (2008). Disposition of Toxic Drugs and Chemicals in Man (8th ed.). Foster City, CA: Biomedical Publications. pp. 388–389.
- ^ "Pyrethroids and Pyrethrins". United States Environmental Protection Agency.
- Fluoroarenes
- Organochlorides
- (cyano-(3-phenoxyphenyl)methyl) 2,2,3-trimethylcyclopropane-1-carboxylates
- Pyrethroids