Cyantraniliprole
| |
| Names | |
|---|---|
| Preferred IUPAC name
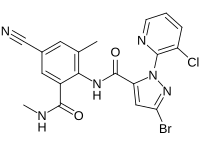
4-Bromo-1-(3-chloropyridin-2-yl)-N-[4-cyano-2-methyl-6-(methylcarbamoyl)phenyl]-1H-pyrazole-5-carboxamide | |
| Other names
Cyazypyr; Exirel
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.205.162 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H14BrClN6O2 | |
| Molar mass | 473.72 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cyantraniliprole is an insecticide of the ryanoid class, specifically a diamide insecticide (IRAC MoA group 28).[1] It is approved for use in the United States, Canada, China, and India.[2] Because of its uncommon mechanism of action as a ryanoid, it has activity against pests such as Diaphorina citri that have developed resistance to other classes of insecticides.[3] Cyantraniliprole is highly toxic to bees, which resulted in registration of its use as a pesticide being delayed in the USA.[4]
References[]
- ^ IRAC International MoA Working Group (March 2020). "IRAC Mode of Action Classification Scheme Version 9.4". Insecticide Resistance Action Committee.
- ^ "Australia to approve DuPont's Exirel insecticide cyantraniliprole". AgroNews. Oct 10, 2013.
- ^ Tiwari S, Stelinski LL (Sep 2013). "Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions". Pest Manag Sci. 69 (9): 1066–1072. doi:10.1002/ps.3468.
- ^ "ICAMA Registration Expert Review Statistics Released - H2 of 2012". 12 December 2012.
External links[]
Categories:
- Insecticides
- Benzamides
- Nitriles
- Pyridines
- Pyrazoles