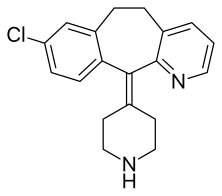
Desloratadine
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Clarinex, Aerius, Allex, others[1][2] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a602002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets, solution) |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Rapidly absorbed |
| Protein binding | 83 to 87% |
| Metabolism | UGT2B10, CYP2C8 |
| Metabolites | 3-Hydroxydesloratadine |
| Onset of action | within 1 hour |
| Elimination half-life | 27 hours |
| Duration of action | up to 24 hours |
| Excretion | 40% as conjugated metabolites into urine Similar amount into the feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.166.554 |
| Chemical and physical data | |
| Formula | C19H19ClN2 |
| Molar mass | 310.83 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Desloratadine (trade names Clarinex and Aerius) is a tricyclic H1 antagonist that is used to treat allergies. It is an active metabolite of loratadine.
It was patented in 1984 and came into medical use in 2001.[3]
Medical uses[]
Desloratadine is used to treat allergic rhinitis, nasal congestion and chronic idiopathic urticaria (hives).[4] It is the major metabolite of loratadine and the two drugs are similar in safety and effectiveness.[4] Desloratadine is available in many dosage forms and under many trade names worldwide.[5]
An emerging indication for desloratadine is in the treatment of acne, as an inexpensive adjuvant to isotretinoin and possibly as maintenance therapy or monotherapy.[6][7]
Side effects[]
The most common side-effects are fatigue (1.2%[8]), dry mouth (3%[8]), and headache (0.6%[8]).[4]
Interactions[]
Co-administration with erythromycin, ketoconazole, azithromycin, fluoxetine or cimetidine resulted in elevated blood plasma concentrations of desloratadine and its metabolite 3-hydroxydesloratadine in studies. However, no clinically relevant changes were observed.[9][10]
Pharmacology[]
Pharmacodynamics[]
Desloratadine is a selective H1-antihistamine which functions as an inverse agonist at the histamine H1 receptor.[11]
At very high doses, is also an antagonist at various subtypes of the muscarinic acetylcholine receptors. This effect is not relevant for the drug's action at therapeutic doses.[12]
Pharmacokinetics[]
Desloratadine is well absorbed from the gut and reaches highest blood plasma concentrations after about three hours. In the bloodstream, 83 to 87% of the substance are bound to plasma proteins.[10]
Desloratadine is metabolized to 3-hydroxydesloratadine in a three-step sequence in normal metabolizers. First, n-glucuronidation of desloratadine by UGT2B10; then, 3-hydroxylation of desloratadine N-glucuronide by CYP2C8; and finally, a non-enzymatic deconjugation of 3-hydroxydesloratadine N-glucuronide.[13] Both desloratadine and 3-hydroxydesloratadine are eliminated via urine and feces with a half-life of 27 hours in normal metabolizers.[10][14]

It exhibits only peripheral activity since it does not readily cross the blood-brain barrier; hence, it does not normally cause drowsiness because it does not readily enter the central nervous system.[15]
Desloratadine does not have a strong effect on a number of tested enzymes in the cytochrome P450 system. It was found to weakly inhibit CYP2B6, CYP2D6, and CYP3A4/CYP3A5, and not to inhibit CYP1A2, CYP2C8, CYP2C9, or CYP2C19. Desloratadine was found to be a potent and relatively selective inhibitor of UGT2B10, a weak to moderate inhibitor of UGT2B17, UGT1A10, and UGT2B4, and not to inhibit UGT1A1, UGT1A3, UGT1A4, UGT1A6, UGT1A9, UGT2B7, UGT2B15, UGT1A7, and UGT1A8.[13]
Pharmacogenomics[]
2% of Caucasian people and 18% of people from African descent are desloratadine poor metabolizers. In these people, the drug reaches threefold highest plasma concentrations six to seven hours after intake, and has a half-life of about 89 hours. However, the safety profile for these subjects is not worse than for extensive (normal) metabolizers.[10][14]
Society and culture[]
Economics[]
When initially released in Canada in 2002 it sold for CA$0.82 per dose.[16]
See also[]
- Benzocycloheptenes
- Azatadine
References[]
- ^ Murdoch, David; Goa, Karen L.; Keam, Susan J. (April 7, 2003). "Desloratadine: an update of its efficacy in the management of allergic disorders". Drugs. 63 (19): 2051–2077. doi:10.2165/00003495-200363190-00010. PMID 12962522.
- ^ "Allex EPAR". European Medicines Agency (EMA).
- ^ Fischer, Jnos; Ganellin, C. Robin (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 549. ISBN 9783527607495.
- ^ a b c See S (2003). "Desloratadine for allergic rhinitis". Am Fam Physician. 68 (10): 2015–6. PMID 14655812.
- ^ Drugs.com Desloratadine entry at drugs.com international Page accessed May 4, 2015
- ^ Lee HE, Chang IK, Lee Y, Kim CD, Seo YJ, Lee JH, Im M (2014). "Effect of antihistamine as an adjuvant treatment of isotretinoin in acne: a randomized, controlled comparative study". J Eur Acad Dermatol Venereol. 28 (12): 1654–60. doi:10.1111/jdv.12403. PMID 25081735. S2CID 3406128.
- ^ Layton AM (2016). "Top Ten List of Clinical Pearls in the Treatment of Acne Vulgaris". Dermatol Clin. 34 (2): 147–57. doi:10.1016/j.det.2015.11.008. PMID 27015774.
- ^ a b c González-Núñez, Vanesa; Valero, Antonio; Mullol, Joaquim (2013-04-11). "Safety evaluation of desloratadine in allergic rhinitis". Expert Opinion on Drug Safety. Informa Healthcare. 12 (3): 445–453. doi:10.1517/14740338.2013.788148. ISSN 1474-0338. PMID 23574541. S2CID 40472187.
- ^ "Clarinex: Prescribing Information" (PDF). U.S. Food and Drug Administration. Retrieved 2022-01-21.
- ^ a b c d "Aerius: EPAR – Product Information" (PDF). European Medicines Agency. Retrieved 2022-01-21.
- ^ Canonica GW, Blaiss M (2011). "Antihistaminic, anti-inflammatory, and antiallergic properties of the nonsedating second-generation antihistamine desloratadine: a review of the evidence". World Allergy Organ J. 4 (2): 47–53. doi:10.1097/WOX.0b013e3182093e19. PMC 3500039. PMID 23268457.
- ^ "Aerius: EPAR – Scientific Discussion" (PDF). European Medicines Agency. 2006-04-03.
- ^ a b Kazmi, F.; Yerino, P.; Barbara, J. E.; Parkinson, A. (2015-07-01). "Further Characterization of the Metabolism of Desloratadine and Its Cytochrome P450 and UDP-glucuronosyltransferase Inhibition Potential: Identification of Desloratadine as a Relatively Selective UGT2B10 Inhibitor". Drug Metabolism and Disposition. 43 (9): 1294–1302. doi:10.1124/dmd.115.065011. ISSN 1521-009X. PMID 26135009.
- ^ a b Drugs.com: Desloratadine Monograph.
- ^ Mann R, Pearce G, Dunn N, Shakir S (2000). "Sedation with "non-sedating" antihistamines: four prescription-event monitoring studies in general practice". BMJ. 320 (7243): 1184–6. doi:10.1136/bmj.320.7243.1184. PMC 27362. PMID 10784544.
- ^ "Report on New Patented Drugs - Aerius". pmprb-cepmb.gc.ca. 13 May 2014. Retrieved 25 November 2019.
External links[]
- "Desloratadine". Drug Information Portal. U.S. National Library of Medicine.
- Benzocycloheptapyridines
- Chloroarenes
- H1 receptor antagonists
- Human drug metabolites
- Peripherally selective drugs
- Piperidines
- Schering-Plough brands
- Merck & Co. brands