Estradiol valerate/megestrol acetate
From Wikipedia, the free encyclopedia
 | |
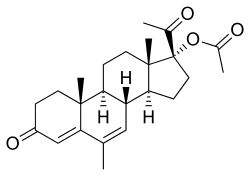 Estradiol valerate (top) and megestrol acetate (bottom) | |
| Combination of | |
|---|---|
| Estradiol valerate | Estrogen |
| Megestrol acetate | Progestogen |
| Clinical data | |
| Other names | EV/MGA |
| Routes of administration | Intramuscular injection (microcapsules) |
Estradiol valerate/megestrol acetate (EV/MGA) is a combined injectable contraceptive which was developed in China in the 1980s but was never marketed.[1][2][3][4][5] It is an aqueous suspension of microcapsules (50–80 μm in diameter) containing 5 mg estradiol valerate (EV) and 15 mg megestrol acetate (MGA).[1][2][4] It was also studied at doses of EV ranging from 0.5 to 5 mg and at doses of MGA ranging from 15 to 25 mg.[3]
See also[]
- List of combined sex-hormonal preparations § Estrogens and progestogens
- Combined injectable birth control § Research
References[]
- ^ a b Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–85. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ a b Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ a b Han ZY, Xiao RQ (August 1984). "Clinical evaluation of intramuscular injection of microencapsulated compound megestrol acetate". Int J Gynaecol Obstet. 22 (4): 319–24. doi:10.1016/0020-7292(84)90091-2. PMID 6152804.
- ^ a b Han ZY, Xiao RQ (June 1985). "A follow-up study of the efficacy and safety of injectable microencapsulated megestrol acetate and a discussion on its contraceptive mechanism". Int J Gynaecol Obstet. 23 (3): 207–11. doi:10.1016/0020-7292(85)90106-7. PMID 2865183.
- ^ Hong Z, Lu B, Wang C (March 1991). "[Determination of megestrol acetate and estradiol valerate in injection of microencapsulated compound megestrol acetate by secondary derivative spectrophotometry]". Hua Xi Yi Ke Da Xue Xue Bao (in Chinese). 22 (1): 87–9. PMID 1774044.
Birth control methods (G02B, G03A) | |||||
|---|---|---|---|---|---|
| Comparison |
| ||||
| Behavioral |
| ||||
| Barrier and / or spermicidal |
| ||||
| Hormonal (formulations) |
| ||||
| Anti-estrogen |
| ||||
| Post-intercourse |
| ||||
| Intrauterine device |
| ||||
| Sterilization |
| ||||
| Experimental |
| ||||
| Long-acting reversible contraception (LARC) |
| ||||
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Androgens |
| ||||||||||||
| Estrogens |
| ||||||||||||
| Progestogens |
| ||||||||||||
Estrogen receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| ER |
| ||||||
| GPER |
| ||||||
| |||||||
Progesterone receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| PR |
| ||||||
| mPR (PAQR) |
| ||||||
| |||||||
Portal: Medicine
Medicine
This drug article relating to the genito-urinary system is a stub. You can help Wikipedia by . |
- v
- t
Retrieved from ""
Categories:
- Abandoned drugs
- Combined injectable contraceptives
- Genito-urinary system drug stubs
Hidden categories:
- CS1 Chinese-language sources (zh)
- Infobox drug articles with non-default infobox title
- Chemical articles without CAS registry number
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs missing an ATC code
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Drugs that are a combination of chemicals
- All stub articles