HIOC
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
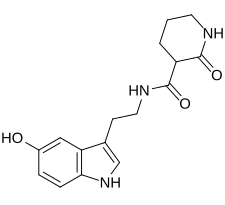
| Formula | C16H19N3O3 |
| Molar mass | 301.346 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
HIOC is a small-molecule agent which acts as a selective TrkB receptor agonist (active at at least 100 nM; prominent activation at 500 nM).[1][2][3] It was derived from N-acetylserotonin (NAS).[2][3][4] Relative to NAS, HIOC possesses greater potency and a longer half-life (~30 min or less for NAS in rats, while HIOC is still detectable up to 24 hours after administration to mice; ~4 hour half-life for HIOC in mouse brain tissues).[2][3] It is described as producing long-lasting activation of the TrkB receptor and downstream signaling kinases associated with the receptor.[2] HIOC is systemically-active and is able to penetrate the blood-brain-barrier.[2] In animal studies, HIOC was found to robustly protect against glutamate-induced excitotoxicity, an action which was TrkB-dependent.[3]
A chemical synthesis of HIOC was published in 2015.[5]
See also[]
- Tropomyosin receptor kinase B § Agonists
References[]
- ^ Longo, Frank M.; Massa, Stephen M. (2013). "Small-molecule modulation of neurotrophin receptors: a strategy for the treatment of neurological disease". Nature Reviews Drug Discovery. 12 (7): 507–525. doi:10.1038/nrd4024. ISSN 1474-1776. PMID 23977697. S2CID 33597483.
- ^ a b c d e Iuvone, P. Michael; Boatright, Jeffrey H.; Tosini, Gianluca; Ye, Keqiang (2014). "N-Acetylserotonin: Circadian Activation of the BDNF Receptor and Neuroprotection in the Retina and Brain". Advances in Experimental Medicine and Biology. 801: 765–771. doi:10.1007/978-1-4614-3209-8_96. ISBN 978-1-4614-3208-1. ISSN 0065-2598. PMC 4069859. PMID 24664769.
- ^ a b c d Shen, J.; Ghai, K.; Sompol, P.; Liu, X.; Cao, X.; Iuvone, P. M.; Ye, K. (2012). "N-acetyl serotonin derivatives as potent neuroprotectants for retinas". Proceedings of the National Academy of Sciences. 109 (9): 3540–3545. doi:10.1073/pnas.1119201109. ISSN 0027-8424. PMC 3295250. PMID 22331903.
- ^ Tosini, G.; Ye, K.; Iuvone, P. M. (2012). "N-Acetylserotonin: Neuroprotection, Neurogenesis, and the Sleepy Brain". The Neuroscientist. 18 (6): 645–653. doi:10.1177/1073858412446634. ISSN 1073-8584. PMC 3422380. PMID 22585341.
- ^ Setterholm NA, McDonald FE, Boatright JH, Iuvone PM (2015). "Gram-scale, chemoselective synthesis of N-[2-(5-hydroxy-1H-indol-3-yl)ethyl]-2-oxopiperidine-3-carboxamide (HIOC)". Tetrahedron Lett. 56 (23): 3413–3415. doi:10.1016/j.tetlet.2015.01.167. PMC 4445863. PMID 26028783.
- Drugs not assigned an ATC code
- Carboxamides
- Tryptamines
- Neuroprotective agents
- Piperidines
- TrkB agonists
- Nervous system drug stubs