Rucaparib
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ruːˈkæpərɪb/ roo-KAP-ər-ib |
| Trade names | Rubraca |
| Other names | AG014699 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a617002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth (tablets) |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 30–45% (Tmax = 1.9 hours) |
| Protein binding | 70% (in vitro) |
| Metabolism | Liver (primarily CYP2D6; 1A2 and 3A4 to a lesser extent) |
| Elimination half-life | 17–19 hours[2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.247.490 |
| Chemical and physical data | |
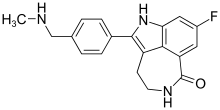
| Formula | C19H18FN3O |
| Molar mass | 323.371 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Rucaparib, sold under the brand name Rubraca, is a PARP inhibitor used as an anti-cancer agent. Rucaparib is a first-in-class pharmaceutical drug targeting the DNA repair enzyme poly-ADP ribose polymerase-1 (PARP-1). It is approved in the United States and in Europe as third line treatment in BRCA-mutated ovarian cancer.[3][4][5]
It can be taken orally in tablet form.[2][6]
The most common side effects include tiredness or weakness, nausea (feeling sick), increased levels of creatinine (which may indicate kidney problems) and liver enzymes in the blood (which may indicate liver damage), vomiting, anaemia (low red blood cell counts), decreased appetite, dysgeusia (taste disturbances), diarrhoea, thrombocytopenia (low levels of platelets) and abdominal pain (belly ache).[7][2]
Medical uses[]
Rucaparib is indicated as monotherapy for the maintenance treatment of adults with platinum-sensitive relapsed high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in response (complete or partial) to platinum-based chemotherapy.[7][2][8]
Rucaparib is indicated as monotherapy treatment of adults with platinum sensitive, relapsed or progressive, BRCA mutated (germline and/or somatic), high-grade epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have been treated with two or more prior lines of platinum based chemotherapy, and who are unable to tolerate further platinum based chemotherapy.[7][2]
Development[]
It was discovered as part of a collaboration between scientists working at the Northern Institute of Cancer Research and Medical School of Newcastle University and Agouron Pharmaceuticals in San Diego, California.[9] It is being developed by Clovis Oncology.
In December 2016, the US Food and Drug Administration (FDA) granted an accelerated approval for use in cases of pretreated advanced ovarian cancer.[10][11]
In the European Union it was designated an orphan medicinal product on 10 October 2012. On 22 March 2018, the Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, recommending the granting of a conditional marketing authorisation, intended for the treatment of relapsed or progressive ovarian cancer.[12][7] It was approved for medical use in the European Union in May 2018.[7]
Pharmacology[]
Mechanism of action[]
Rucaparib inhibits "the contraction of isolated vascular smooth muscle, including that from the tumours of cancer patients. It also reduces the migration of some cancer and normal cells in culture."[13]
As a PARP inhibitor, rucaparib is expected to be more effective in the 9% of pancreatic cancers with a BRCA mutation (BRCA1 or BRCA2).[14]
Clinical trials[]
After the FDA approval, TRITON2 and TRITON3 mCRPC studies were initiated in order to determine how patients with prostate cancer will respond to the rucaparib drug. The studies for these two trials are still going on and the estimated dates for the first results are range between 2019 and 2022.[15]
The ARIEL3 and ARIEL4 are two randomized, double-blind phase III studies. The ARIEL3 study was designed to evaluate the effect of the investigational agent as a maintenance treatment for the advanced platinum-sensitive ovarian cancer patients compared placebo after their response to at least two prior chemotherapies. The top-line results from the study were presented at the ESMO 2017 congress and right after that, it was published in the Lancet journal in September 2017. The findings showed significant improvement in progression-free survival (PFS) in patients treated with Rubraca than placebo. Recently, in October 2017, a supplemental sNDA for the rucaparib ARIEL3 maintenance treatment has been submitted to the FDA.[16]
The ARIEL4 trial is still ongoing[when?] to evaluate how patients will best respond to treatment with rucaparib compared with chemotherapy. The estimated data collection date for primary outcome measurement will be in June 2022.[17]
See also[]
References[]
- ^ "Rubraca 200mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). 19 June 2019. Retrieved 17 May 2020.
- ^ a b c d e "Rubraca- rucaparib tablet, film coated". DailyMed. 6 April 2018. Retrieved 17 May 2020.
- ^ Colombo I, Lheureux S, Oza AM (2018). "BRCA advanced ovarian cancer". Drug Design, Development and Therapy. 12: 605–617. doi:10.2147/DDDT.S130809. PMC 5868608. PMID 29606854.
- ^ Musella A, Bardhi E, Marchetti C, Vertechy L, Santangelo G, Sassu C, et al. (May 2018). "Rucaparib: An emerging parp inhibitor for treatment of recurrent ovarian cancer". Cancer Treatment Reviews. 66: 7–14. doi:10.1016/j.ctrv.2018.03.004. PMID 29605737.
- ^ Shirley M (April 2019). "Rucaparib: A Review in Ovarian Cancer". Targeted Oncology. 14 (2): 237–246. doi:10.1007/s11523-019-00629-5. PMID 30830551. S2CID 71147857.
- ^ "Cancer Research Launches New Drug Trial". netdoctor.co.uk. Hearst Magazines UK. 10 January 2012. Retrieved 20 December 2016.
- ^ a b c d e "Rubraca EPAR". European Medicines Agency (EMA). Retrieved 17 May 2020. Text was copied from this source which is © European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ "FDA approves rucaparib". U.S. Food and Drug Administration (FDA) (Press release). 6 April 2018. Retrieved 17 May 2020.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ^ White AW, Almassy R, Calvert AH, Curtin NJ, Griffin RJ, Hostomsky Z, et al. (November 2000). "Resistance-modifying agents. 9. Synthesis and biological properties of benzimidazole inhibitors of the DNA repair enzyme poly(ADP-ribose) polymerase". Journal of Medicinal Chemistry. 43 (22): 4084–97. doi:10.1021/jm000950v. PMID 11063605.
- ^ Bankhead C (19 December 2016). "PARP Inhibitor Gets FDA Nod for Ovarian Cancer". MedPage Today, LLC. Retrieved 20 December 2016.
- ^ "Rubraca (rucaparib) Tablets". U.S. Food and Drug Administration (FDA). 30 January 2017. Retrieved 17 May 2020.
- ^ "Rubraca". European Medical Agency. 22 March 2018. Archived from the original on 29 May 2018.
- ^ "Does the PARP inhibitor AG014699 act via the nucleotide P2 receptor?" (PDF). School of Pharmacy - PhD Projects 2009. Archived from the original (PDF) on 13 June 2011. Retrieved 17 November 2009.
- ^ "Rucaparib shows clinical benefit in pancreatic cancer patients with BRCA mutation". sciencedaily.com.
- ^ ""Rucaparib Clinical Overview" (PDF). Clovis Oncology. Archived from the original (PDF) on 13 April 2018.
- ^ Coleman RL, Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, et al. (October 2017). "Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): a randomised, double-blind, placebo-controlled, phase 3 trial". Lancet. 390 (10106): 1949–1961. doi:10.1016/S0140-6736(17)32440-6. PMC 5901715. PMID 28916367.
- ^ "Ledermann J, Oza AM, Lorusso D (November 2017). "ARIEL3: a phase 3, randomised, double-blind study of rucaparib vs placebo following response to platinum-based chemotherapy for recurrent ovarian carcinoma (OC)" (PDF): 28. Cite journal requires
|journal=(help)
External links[]
- "Rucaparib". Drug Information Portal. U.S. National Library of Medicine.
- Clinical trial number NCT01968213 for "A Study of Rucaparib as Switch Maintenance Following Platinum-Based Chemotherapy in Patients With Platinum-Sensitive, High-Grade Serous or Endometrioid Epithelial Ovarian, Primary Peritoneal or Fallopian Tube Cancer (ARIEL3) " at ClinicalTrials.gov
- Benzazepines
- Cancer treatments
- Tryptamines
- Lactams
- PARP inhibitors
- Fluoroarenes
- Orphan drugs