CYP2D6
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the CYP2D6 gene. CYP2D6 is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra.
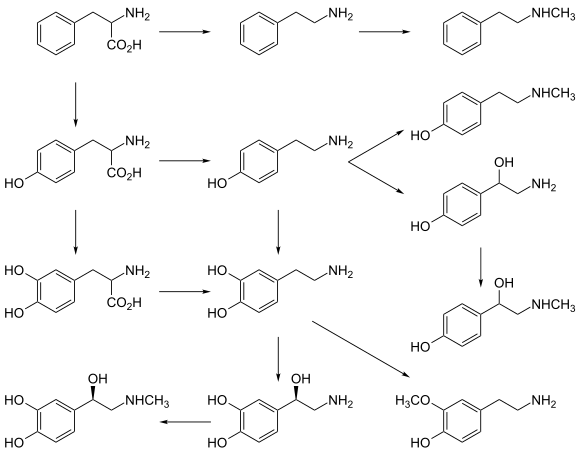
CYP2D6, a member of the cytochrome P450 mixed-function oxidase system, is one of the most important enzymes involved in the metabolism of xenobiotics in the body. In particular, CYP2D6 is responsible for the metabolism and elimination of approximately 25% of clinically used drugs, via the addition or removal of certain functional groups – specifically, hydroxylation, demethylation, and dealkylation.[3] CYP2D6 also activates some prodrugs. This enzyme also metabolizes several endogenous substances, such as hydroxytryptamines, neurosteroids, and both m-tyramine and p-tyramine which CYP2D6 metabolizes into dopamine in the brain and liver.[3][4]
Considerable variation exists in the efficiency and amount of CYP2D6 enzyme produced between individuals. Hence, for drugs that are metabolized by CYP2D6 (that is, are CYP2D6 substrates), certain individuals will eliminate these drugs quickly (ultrarapid metabolizers) while others slowly (poor metabolizers). If a drug is metabolized too quickly, it may decrease the drug's efficacy while if the drug is metabolized too slowly, toxicity may result.[5] So, the dose of the drug may have to be adjusted to take into account of the speed at which it is metabolized by CYP2D6.[6]
Other drugs may function as inhibitors of CYP2D6 activity or inducers of CYP2D6 enzyme expression that will lead to decreased or increased CYP2D6 activity respectively. If such a drug is taken at the same time as a second drug that is a CYP2D6 substrate, the first drug may affect the elimination rate of the second through what is known as a drug-drug interaction.[5]
Gene[]
The gene is located on chromosome 22q13.1. near two cytochrome P450 pseudogenes (CYP2D7P and CYP2D8P).[7] Among them, CYP2D7P originated from CYP2D6 in a stem lineage of great apes and humans,[8] the CYP2D8P originated from CYP2D6 in a stem lineage of Catarrhine and New World monkeys' stem lineage.[9] Alternatively spliced transcript variants encoding different isoforms have been found for this gene.[10]
Genotype/phenotype variability[]
CYP2D6 shows the largest phenotypical variability among the CYPs, largely due to genetic polymorphism. The genotype accounts for normal, reduced, and non-existent CYP2D6 function in subjects. Pharmacogenomic tests are now available to identify patients with variations in the CYP2D6 allele and have been shown to have widespread use in clinical practice.[11] The CYP2D6 function in any particular subject may be described as one of the following:[12]
- poor metabolizer – little or no CYP2D6 function
- intermediate metabolizers – metabolize drugs at a rate somewhere between the poor and extensive metabolizers
- extensive metabolizer – normal CYP2D6 function
- ultrarapid metabolizer – multiple copies of the CYP2D6 gene are expressed, so greater-than-normal CYP2D6 function occurs
A patient's CYP2D6 phenotype is often clinically determined via the administration of debrisoquine (a selective CYP2D6 substrate) and subsequent plasma concentration assay of the debrisoquine metabolite (4-hydroxydebrisoquine).[13]
The type of CYP2D6 function of an individual may influence the person's response to different doses of drugs that CYP2D6 metabolizes. The nature of the effect on the drug response depends not only on the type of CYP2D6 function, but also on the extent to which processing of the drug by CYP2D6 results in a chemical that has an effect that is similar, stronger, or weaker than the original drug, or no effect at all. For example, if CYP2D6 converts a drug that has a strong effect into a substance that has a weaker effect, then poor metabolizers (weak CYP2D6 function) will have an exaggerated response to the drug and stronger side-effects; conversely, if CYP2D6 converts a different drug into a substance that has a greater effect than its parent chemical, then ultrarapid metabolizers (strong CYP2D6 function) will have an exaggerated response to the drug and stronger side-effects.[14]
Genetic basis of variability[]
The genetic basis for CYP2D6-mediated metabolic variability is the CYP2D6 allele, located on chromosome 22. Subjects possessing certain allelic variants will show normal, decreased, or no CYP2D6 function, depending on the allele. Pharmacogenomic tests are now available to identify patients with variations in the CYP2D6 allele and have been shown to have widespread use in clinical practice.[11] The current known alleles of CYP2D6 and their clinical function can be found in databases such as PharmVar.[15]
| CYP2D6 enzyme activity for selected alleles[16][15] | |
| Allele | CYP2D6 activity |
| CYP2D6*1 | normal |
| CYP2D6*2 | normal |
| CYP2D6*3 | none |
| CYP2D6*4 | none |
| CYP2D6*5 | none |
| CYP2D6*6 | none |
| CYP2D6*7 | none |
| CYP2D6*8 | none |
| CYP2D6*9 | decreased |
| CYP2D6*10 | decreased |
| CYP2D6*11 | none |
| CYP2D6*12 | none |
| CYP2D6*13 | none |
| CYP2D6*14 | none |
| CYP2D6*15 | none |
| CYP2D6*17 | decreased |
| CYP2D6*19 | none |
| CYP2D6*20 | none |
| CYP2D6*21 | none |
| CYP2D6*29 | decreased |
| CYP2D6*31 | none |
| CYP2D6*38 | none |
| CYP2D6*40 | none |
| CYP2D6*41 | decreased |
| CYP2D6*42 | none |
| CYP2D6*44 | none |
| CYP2D6*47 | none |
| CYP2D6*50 | decreased |
| CYP2D6*51 | none |
| CYP2D6*68 | none |
| CYP2D6*92 | none |
| CYP2D6*100 | none |
| CYP2D6*101 | none |
| CYP2D6 duplication | increased |
Ethnic factors in variability[]
Ethnicity is a factor in the occurrence of CYP2D6 variability. The lack of the liver cytochrome CYP2D6 enzyme occurs approximately in 7–10% in white populations, and is lower in most other ethnic groups such as Asians and African-Americans at 2% each.[17] The occurrence of CYP2D6 ultrarapid metabolizers appears to be greater among Middle Eastern and North African populations.[18]
Caucasians with European descent predominantly (around 71%) have the functional group of CYP2D6 alleles, while functional alleles represent only around 50% of the allele frequency in populations of Asian descent.[19]
This variability is accounted for by the differences in the prevalence of various CYP2D6 alleles among the populations–approximately 10% of whites are intermediate metabolizers, due to decreased CYP2D6 function, because they appear to have the non-functional CYP2D6*4 allele,[16] while approximately 50% of Asians possess the decreased functioning CYP2D6*10 allele.[16]
Ligands[]
Following is a table of selected substrates, inducers and inhibitors of CYP2D6. Where classes of agents are listed, there may be exceptions within the class.
Inhibitors of CYP2D6 can be classified by their potency, such as:
- Strong inhibitor being one that causes at least a 5-fold increase in the plasma AUC values of sensitive substrates metabolized through CYP2D6, or more than 80% decrease in clearance thereof.[20]
- Moderate inhibitor being one that causes at least a 2-fold increase in the plasma AUC values of sensitive substrates metabolized through CYP2D6, or 50-80% decrease in clearance thereof.[20]
- Weak inhibitor being one that causes at least a 1.25-fold but less than 2-fold increase in the plasma AUC values of sensitive substrates metabolized through CYP2D6, or 20-50% decrease in clearance thereof.[20]
| Substrates ↑ = bioactivation by CYP2D6 |
Inhibitors | Inducers |
|---|---|---|
|
Strong
Moderate Weak
Unspecified potency
|
Strong
Unspecified potency |
Dopamine biosynthesis[]
References[]
- ^ a b c ENSG00000275211, ENSG00000280905, ENSG00000282966, ENSG00000283284, ENSG00000272532 GRCh38: Ensembl release 89: ENSG00000100197, ENSG00000275211, ENSG00000280905, ENSG00000282966, ENSG00000283284, ENSG00000272532 - Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b Wang B, Yang LP, Zhang XZ, Huang SQ, Bartlam M, Zhou SF (2009). "New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme". Drug Metabolism Reviews. 41 (4): 573–643. doi:10.1080/03602530903118729. PMID 19645588. S2CID 41857580.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–8. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ a b Teh LK, Bertilsson L (2012). "Pharmacogenomics of CYP2D6: molecular genetics, interethnic differences and clinical importance". Drug Metabolism and Pharmacokinetics. 27 (1): 55–67. doi:10.2133/dmpk.DMPK-11-RV-121. PMID 22185816.
- ^ Walko CM, McLeod H (April 2012). "Use of CYP2D6 genotyping in practice: tamoxifen dose adjustment". Pharmacogenomics. 13 (6): 691–7. doi:10.2217/pgs.12.27. PMID 22515611.
- ^ Ahmad, HI; Afzal, G; Jamal, A; Kiran, S; Khan, MA; Mehmood, K; Kamran, Z; Ahmed, I; Ahmad, S; Ahmad, A; Hussain, J; Almas, S (2021). "In Silico Structural, Functional, and Phylogenetic Analysis of Cytochrome (CYPD) Protein Family". BioMed Research International. 2021: 5574789. doi:10.1155/2021/5574789. PMC 8128545. PMID 34046497.
- ^ Wang, H; Tompkins, LM (September 2008). "CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme". Current Drug Metabolism. 9 (7): 598–610. doi:10.2174/138920008785821710. PMC 2605793. PMID 18781911.
- ^ Yasukochi, Y; Satta, Y (2011). "Evolution of the CYP2D gene cluster in humans and four non-human primates". Genes & Genetic Systems. 86 (2): 109–16. doi:10.1266/ggs.86.109. PMID 21670550.
- ^ "Entrez Gene: CYP2D6 cytochrome P450, family 2, subfamily D, polypeptide 6".
- ^ a b Dinama O, Warren AM, Kulkarni J (August 2014). "The role of pharmacogenomic testing in psychiatry: Real world examples". The Australian and New Zealand Journal of Psychiatry. 48 (8): 778. doi:10.1177/0004867413520050. PMID 24413808. S2CID 206399446.
- ^ Bertilsson L, Dahl ML, Dalén P, Al-Shurbaji A (February 2002). "Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs". British Journal of Clinical Pharmacology. 53 (2): 111–22. doi:10.1046/j.0306-5251.2001.01548.x. PMC 1874287. PMID 11851634.
- ^ Llerena A, Dorado P, Peñas-Lledó EM (January 2009). "Pharmacogenetics of debrisoquine and its use as a marker for CYP2D6 hydroxylation capacity". Pharmacogenomics. 10 (1): 17–28. doi:10.2217/14622416.10.1.17. PMID 19102711.
- ^ Lynch T, Price A (August 2007). "The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects". American Family Physician. 76 (3): 391–6. PMID 17708140.
- ^ a b "PharmVar". Retrieved 20 May 2020.
- ^ a b c Droll K, Bruce-Mensah K, Otton SV, Gaedigk A, Sellers EM, Tyndale RF (August 1998). "Comparison of three CYP2D6 probe substrates and genotype in Ghanaians, Chinese and Caucasians". Pharmacogenetics. 8 (4): 325–33. doi:10.1097/00008571-199808000-00006. PMID 9731719.
- ^ Lilley LL, Harrington S, Snyder JS, Swart B (2007). Pharmacology and the Nursing Process. Toronto: Mosby Elsevier. p. 25. ISBN 9780779699711.
- ^ McLellan RA, Oscarson M, Seidegård J, Evans DA, Ingelman-Sundberg M (June 1997). "Frequent occurrence of CYP2D6 gene duplication in Saudi Arabians". Pharmacogenetics. 7 (3): 187–91. doi:10.1097/00008571-199706000-00003. PMID 9241658.
- ^ Bradford LD (March 2002). "CYP2D6 allele frequency in European Caucasians, Asians, Africans and their descendants". Pharmacogenomics. 3 (2): 229–43. doi:10.1517/14622416.3.2.229. PMID 11972444.
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag ah ai aj ak al am an ao ap aq ar as at au av aw ax ay az ba bb bc bd be bf bg bh bi bj bk bl bm bn bo bp bq br bs bt bu bv bw bx by bz ca cb cc cd ce cf cg ch Flockhart DA (2007). "Drug Interactions: Cytochrome P450 Drug Interaction Table". Indiana University School of Medicine. Retrieved in July 2011
- ^ a b c d e f g h i j k l m n o p q r s t u v w x y z aa ab ac ad ae af ag FASS (drug formulary): Swedish environmental classification of pharmaceuticals Facts for prescribers (Fakta för förskrivare), retrieved July 2011
- ^ a b Leeder JS (June 2001). "Pharmacogenetics and pharmacogenomics". Pediatric Clinics of North America. 48 (3): 765–81. doi:10.1016/S0031-3955(05)70338-2. PMID 11411304.
- ^ "Hydrocodone". Drugbank. Retrieved 14 June 2011.
- ^ Hoskins JM, Carey LA, McLeod HL (August 2009). "CYP2D6 and tamoxifen: DNA matters in breast cancer". Nature Reviews. Cancer. 9 (8): 576–86. doi:10.1038/nrc2683. PMID 19629072. S2CID 19501089.
- ^ a b c d e Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ Vizeli P, Straumann I, Holze F, Schmid Y, Dolder PC, Liechti ME (May 2021). "Genetic influence of CYP2D6 on pharmacokinetics and acute subjective effects of LSD in a pooled analysis". Scientific Reports. 11 (1): 10851. Bibcode:2021NatSR..1110851V. doi:10.1038/s41598-021-90343-y. PMC 8149637. PMID 34035391.
- ^ a b "DILTIAZEM HCL CD- diltiazem hydrochloride capsule, coated, extended release". DailyMed. 2017-02-01. Retrieved 2019-01-31.
- ^ "NIFEDIPINE EXTENDED RELEASE- nifedipine tablet, extended release". DailyMed. 2012-11-29. Retrieved 2019-02-01.
- ^ Kotlyar M, Brauer LH, Tracy TS, Hatsukami DK, Harris J, Bronars CA, Adson DE (June 2005). "Inhibition of CYP2D6 activity by bupropion". Journal of Clinical Psychopharmacology. 25 (3): 226–9. doi:10.1097/01.jcp.0000162805.46453.e3. PMID 15876900. S2CID 24591644.
- ^ Fasinu PS, Tekwani BL, Avula B, Chaurasiya ND, Nanayakkara NP, Wang YH, et al. (September 2016). "Pathway-specific inhibition of primaquine metabolism by chloroquine/quinine". Malaria Journal. 15: 466. doi:10.1186/s12936-016-1509-x. PMC 5020452. PMID 27618912.
- ^ "Medical Cannabis Adverse Effects & Drug Interactions" (PDF).
- ^ Zhang W, Ramamoorthy Y, Tyndale RF, Sellers EM (June 2003). "Interaction of buprenorphine and its metabolite norbuprenorphine with cytochromes p450 in vitro". Drug Metabolism and Disposition. 31 (6): 768–72. doi:10.1124/dmd.31.6.768. PMID 12756210.
- ^ a b "Citalopram Oral Solution". Drugs.com.
- ^ "Escitalopram-drug-information". UpToDate. Retrieved 2019-05-22.
- ^ a b "Drug Development and Drug Interactions: Table of Substrates, Inhibitors and Inducers". FDA. 26 May 2021.
- ^ Nevels RM, Weiss NH, Killebrew AE, Gontkovsky ST (July 2013). "Methylphenidate and Its Under-recognized, Under- explained, and Serious Drug Interactions: A Review of the Literature with Heightened Concerns" (PDF). German Journal of Psychiatry: 29–42.
- ^ Bailey DG, Bend JR, Arnold JM, Tran LT, Spence JD (July 1996). "Erythromycin-felodipine interaction: magnitude, mechanism, and comparison with grapefruit juice". Clinical Pharmacology and Therapeutics. 60 (1): 25–33. doi:10.1016/s0009-9236(96)90163-0. PMID 8689808. S2CID 1246705.
- ^ Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, et al. (May 1997). "Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression". The Journal of Clinical Investigation. 99 (10): 2545–53. doi:10.1172/jci119439. PMC 508096. PMID 9153299.
- ^ Guengerich FP, Brian WR, Iwasaki M, Sari MA, Bäärnhielm C, Berntsson P (June 1991). "Oxidation of dihydropyridine calcium channel blockers and analogues by human liver cytochrome P-450 IIIA4". Journal of Medicinal Chemistry. 34 (6): 1838–44. doi:10.1021/jm00110a012. PMID 2061924.
- ^ Owen JR, Nemeroff CB (1998-05-30). "New antidepressants and the cytochrome P450 system: focus on venlafaxine, nefazodone, and mirtazapine". Depression and Anxiety. 7 Suppl 1 (SUPPL. 1): 24–32. doi:10.1002/(SICI)1520-6394(1998)7:1+<24::AID-DA7>3.0.CO;2-F. PMID 9597349.
- ^ a b c d FASS, The Swedish official drug catalog > Kodein Recip Last reviewed 2008-04-08
- ^ He N, Zhang WQ, Shockley D, Edeki T (February 2002). "Inhibitory effects of H1-antihistamines on CYP2D6- and CYP2C9-mediated drug metabolic reactions in human liver microsomes". European Journal of Clinical Pharmacology. 57 (12): 847–51. doi:10.1007/s00228-001-0399-0. PMID 11936702. S2CID 601644.
- ^ Zhao Y, Hellum BH, Liang A, Nilsen OG (June 2015). "Inhibitory Mechanisms of Human CYPs by Three Alkaloids Isolated from Traditional Chinese Herbs". Phytotherapy Research. 29 (6): 825–34. doi:10.1002/ptr.5285. PMID 25640685. S2CID 24002845.
- ^ Hermann R, von Richter O (September 2012). "Clinical evidence of herbal drugs as perpetrators of pharmacokinetic drug interactions". Planta Medica. 78 (13): 1458–77. doi:10.1055/s-0032-1315117. PMID 22855269.
- ^ Feng P, Zhao L, Guo F, Zhang B, Fang L, Zhan G, Xu X, Fang Q, Liang Z, Li B (September 2018). "The enhancement of cardiotoxicity that results from inhibiton of CYP 3A4 activity and hERG channel by berberine in combination with statins". Chemico-Biological Interactions. 293: 115–123. doi:10.1016/j.cbi.2018.07.022. PMID 30086269.
- ^ Foster BC, Sockovie ER, Vandenhoek S, Bellefeuille N, Drouin CE, Krantis A, et al. (2008). "In Vitro Activity of St. John's Wort Against Cytochrome P450 Isozymes and P-Glycoprotein". Pharmaceutical Biology. 42 (2): 159–69. doi:10.1080/13880200490512034. S2CID 2366709.
- ^ Gaudineau, Cédric; Auclair, Karine (May 2004). "Inhibition of human P450 enzymes by nicotinic acid and nicotinamide". Biochemical and Biophysical Research Communications. 317 (3): 950–956. doi:10.1016/j.bbrc.2004.03.137. PMID 15081432.
- ^ Briguglio M, Hrelia S, Malaguti M, Serpe L, Canaparo R, Dell'Osso B, et al. (December 2018). "Food Bioactive Compounds and Their Interference in Drug Pharmacokinetic/Pharmacodynamic Profiles". Pharmaceutics. 10 (4): 277. doi:10.3390/pharmaceutics10040277. PMC 6321138. PMID 30558213.
- ^ Kudo S, Ishizaki T (December 1999). "Pharmacokinetics of haloperidol: an update". Clinical Pharmacokinetics. 37 (6): 435–56. doi:10.2165/00003088-199937060-00001. PMID 10628896. S2CID 71360020.
- ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
Further reading[]
- Smith G, Stubbins MJ, Harries LW, Wolf CR (December 1998). "Molecular genetics of the human cytochrome P450 monooxygenase superfamily". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 28 (12): 1129–65. doi:10.1080/004982598238868. PMID 9890157.
- Wolf CR, Smith G (1999). "Cytochrome P450 CYP2D6". IARC Scientific Publications (148): 209–29. PMID 10493260.
- Ding X, Kaminsky LS (2003). "Human extrahepatic cytochromes P450: function in xenobiotic metabolism and tissue-selective chemical toxicity in the respiratory and gastrointestinal tracts". Annual Review of Pharmacology and Toxicology. 43: 149–73. doi:10.1146/annurev.pharmtox.43.100901.140251. PMID 12171978.
- Lilienfeld S (2006). "Galantamine--a novel cholinergic drug with a unique dual mode of action for the treatment of patients with Alzheimer's disease". CNS Drug Reviews. 8 (2): 159–76. doi:10.1111/j.1527-3458.2002.tb00221.x. PMC 6741688. PMID 12177686.
- Yu AM, Idle JR, Gonzalez FJ (May 2004). "Polymorphic cytochrome P450 2D6: humanized mouse model and endogenous substrates". Drug Metabolism Reviews. 36 (2): 243–77. doi:10.1081/DMR-120034000. PMID 15237854. S2CID 11330784.
- Abraham JE, Maranian MJ, Driver KE, Platte R, Kalmyrzaev B, Baynes C, et al. (2010). "CYP2D6 gene variants: association with breast cancer specific survival in a cohort of breast cancer patients from the United Kingdom treated with adjuvant tamoxifen". Breast Cancer Research. 12 (4): R64. doi:10.1186/bcr2629. PMC 2949659. PMID 20731819.
- Abraham JE, Maranian MJ, Driver KE, Platte R, Kalmyrzaev B, Baynes C, et al. (June 2011). "CYP2D6 gene variants and their association with breast cancer susceptibility". Cancer Epidemiology, Biomarkers & Prevention. 20 (6): 1255–8. doi:10.1158/1055-9965.EPI-11-0321. PMID 21527579. S2CID 32846974.
External links[]
- Flockhart Lab Cyp2D6 Substrates Page at IUPUI
- PharmGKB: Annotated PGx Gene Information for CYP2D6
- Human CYP2D6 genome location and CYP2D6 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P10635 (Cytochrome P450 2D6) at the PDBe-KB.
- Genes on human chromosome 22
- Cytochrome P450
- EC 1.14.14
- Amphetamine