CYP27A1
| CYP27A1 | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||
| Aliases | CYP27A1, CP27, CTX, CYP27, cytochrome P450 family 27 subfamily A member 1 | ||||||||||||||||||||||||
| External IDs | OMIM: 606530 MGI: 88594 HomoloGene: 36040 GeneCards: CYP27A1 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Orthologs | |||||||||||||||||||||||||
| Species | Human | Mouse | |||||||||||||||||||||||
| Entrez | |||||||||||||||||||||||||
| Ensembl | |||||||||||||||||||||||||
| UniProt | |||||||||||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||||||||||
| RefSeq (protein) | |||||||||||||||||||||||||
| Location (UCSC) | Chr 2: 218.78 – 218.82 Mb | Chr 1: 74.75 – 74.78 Mb | |||||||||||||||||||||||
| PubMed search | [3] | [4] | |||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
CYP27A1 is a gene encoding a cytochrome P450 oxidase, and is commonly known as sterol 27-hydroxylase. This enzyme is located in many different tissues where it is found within the mitochondria. It is most prominently involved in the biosynthesis of bile acids.
Function[]
CYP27A1 participates in the degradation of cholesterol to bile acids in both the classic and acidic pathways.[5] It is the initiating enzyme in the acidic pathway to bile acids, yielding oxysterols by introducing a hydroxyl group to the carbon at the 27 position in cholesterol. In the acidic pathway, it produces 27-hydroxycholesterol from cholesterol whereas in the classic or neutral pathway, it produces .
While CYP27A1 is present in many different tissues, its function in these tissues is largely uncharacterized. In macrophages, 27-hydroxycholesterol generated by this enzyme may be helpful against the production of inflammatory factors associated with cardiovascular disease.[6]
Clinical significance[]
Mutations in CYP27A1 are associated with cerebrotendineous xanthomatosis, a rare lipid storage disease.
Inhibitors of CYP27A1 may be effective as adjuvants in the treatment of ER-positive breast cancer due to inhibition of the production of 27-hydroxycholesterol (which has estrogenic actions and stimulates the growth of ER-positive breast cancer cells).[7] Some marketed drugs that have been identified as CYP27A1 inhibitors include anastrozole, fadrozole, bicalutamide, dexmedetomidine, ravuconazole, and posaconazole.[7]
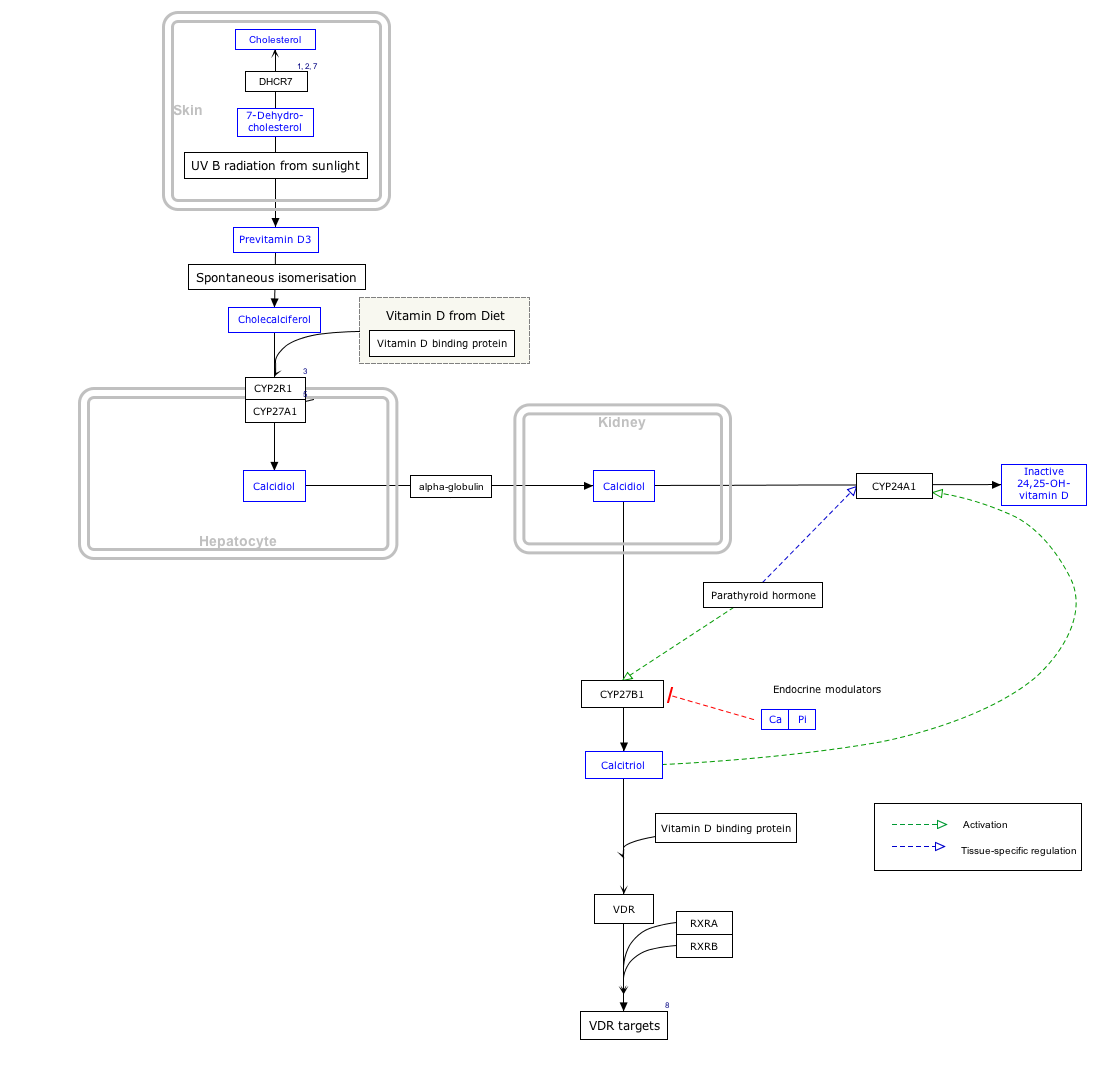
Interactive pathway map[]
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
- ^ The interactive pathway map can be edited at WikiPathways: "VitaminDSynthesis_WP1531".
See also[]
References[]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000135929 - Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000026170 - Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Chiang JY (February 1998). "Regulation of bile acid synthesis". Frontiers in Bioscience. 3 (4): d176-93. doi:10.2741/a273. PMID 9450986.
- ^ Taylor JM, Borthwick F, Bartholomew C, Graham A (June 2010). "Overexpression of steroidogenic acute regulatory protein increases macrophage cholesterol efflux to apolipoprotein AI". Cardiovascular Research. 86 (3): 526–34. doi:10.1093/cvr/cvq015. PMID 20083572.
- ^ a b Mast N, Lin JB, Pikuleva IA (September 2015). "Marketed Drugs Can Inhibit Cytochrome P450 27A1, a Potential New Target for Breast Cancer Adjuvant Therapy". Molecular Pharmacology. 88 (3): 428–36. doi:10.1124/mol.115.099598. PMC 4551053. PMID 26082378.
Further reading[]
- Cali JJ, Russell DW (April 1991). "Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis". The Journal of Biological Chemistry. 266 (12): 7774–8. doi:10.1016/S0021-9258(20)89517-9. PMID 1708392.
- Cali JJ, Hsieh CL, Francke U, Russell DW (April 1991). "Mutations in the bile acid biosynthetic enzyme sterol 27-hydroxylase underlie cerebrotendinous xanthomatosis". The Journal of Biological Chemistry. 266 (12): 7779–83. doi:10.1016/S0021-9258(20)89518-0. PMC 4449724. PMID 2019602.
- Guo YD, Strugnell S, Back DW, Jones G (September 1993). "Transfected human liver cytochrome P-450 hydroxylates vitamin D analogs at different side-chain positions". Proceedings of the National Academy of Sciences of the United States of America. 90 (18): 8668–72. Bibcode:1993PNAS...90.8668G. doi:10.1073/pnas.90.18.8668. PMC 47419. PMID 7690968.
- Kim KS, Kubota S, Kuriyama M, Fujiyama J, Björkhem I, Eggertsen G, Seyama Y (June 1994). "Identification of new mutations in sterol 27-hydroxylase gene in Japanese patients with cerebrotendinous xanthomatosis (CTX)". Journal of Lipid Research. 35 (6): 1031–9. doi:10.1016/S0022-2275(20)40096-3. PMID 7915755.
- Maruyama K, Sugano S (January 1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Leitersdorf E, Reshef A, Meiner V, Levitzki R, Schwartz SP, Dann EJ, Berkman N, Cali JJ, Klapholz L, Berginer VM (June 1993). "Frameshift and splice-junction mutations in the sterol 27-hydroxylase gene cause cerebrotendinous xanthomatosis in Jews or Moroccan origin". The Journal of Clinical Investigation. 91 (6): 2488–96. doi:10.1172/JCI116484. PMC 443309. PMID 8514861.
- Chen W, Kubota S, Kim KS, Cheng J, Kuriyama M, Eggertsen G, Björkhem I, Seyama Y (May 1997). "Novel homozygous and compound heterozygous mutations of sterol 27-hydroxylase gene (CYP27) cause cerebrotendinous xanthomatosis in three Japanese patients from two unrelated families". Journal of Lipid Research. 38 (5): 870–9. doi:10.1016/S0022-2275(20)37212-6. PMID 9186905.
- Reiss AB, Martin KO, Rojer DE, Iyer S, Grossi EA, Galloway AC, Javitt NB (June 1997). "Sterol 27-hydroxylase: expression in human arterial endothelium". Journal of Lipid Research. 38 (6): 1254–60. doi:10.1016/S0022-2275(20)37206-0. PMID 9215552.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, Suyama A, Sugano S (October 1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Chen W, Kubota S, Ujike H, Ishihara T, Seyama Y (October 1998). "A novel Arg362Ser mutation in the sterol 27-hydroxylase gene (CYP27): its effects on pre-mRNA splicing and enzyme activity". Biochemistry. 37 (43): 15050–6. doi:10.1021/bi9807660. PMID 9790667.
- Shiga K, Fukuyama R, Kimura S, Nakajima K, Fushiki S (November 1999). "Mutation of the sterol 27-hydroxylase gene (CYP27) results in truncation of mRNA expressed in leucocytes in a Japanese family with cerebrotendinous xanthomatosis". Journal of Neurology, Neurosurgery, and Psychiatry. 67 (5): 675–7. doi:10.1136/jnnp.67.5.675. PMC 1736608. PMID 10519880.
- Gascon-Barré M, Demers C, Ghrab O, Theodoropoulos C, Lapointe R, Jones G, Valiquette L, Ménard D (January 2001). "Expression of CYP27A, a gene encoding a vitamin D-25 hydroxylase in human liver and kidney". Clinical Endocrinology. 54 (1): 107–15. doi:10.1046/j.1365-2265.2001.01160.x. PMID 11167933. S2CID 21864150.
- Johnston TP, Nguyen LB, Chu WA, Shefer S (October 2001). "Potency of select statin drugs in a new mouse model of hyperlipidemia and atherosclerosis". International Journal of Pharmaceutics. 229 (1–2): 75–86. doi:10.1016/S0378-5173(01)00834-1. PMID 11604260.
- Toba H, Fukuyama R, Sasaki M, Shiga K, Ishibashi S, Fushiki S (January 2002). "A Japanese patient with cerebrotendinous xanthomatosis has different mutations within two functional domains of CYP27". Clinical Genetics. 61 (1): 77–8. doi:10.1034/j.1399-0004.2002.610116.x. PMID 11903362. S2CID 43642764.
- Lamon-Fava S, Schaefer EJ, Garuti R, Salen G, Calandra S (March 2002). "Two novel mutations in the sterol 27-hydroxylase gene causing cerebrotendinous xanthomatosis". Clinical Genetics. 61 (3): 185–91. doi:10.1034/j.1399-0004.2002.610303.x. PMID 12000359. S2CID 45930401.
- Björkhem I, Araya Z, Rudling M, Angelin B, Einarsson C, Wikvall K (July 2002). "Differences in the regulation of the classical and the alternative pathway for bile acid synthesis in human liver. No coordinate regulation of CYP7A1 and CYP27A1". The Journal of Biological Chemistry. 277 (30): 26804–7. doi:10.1074/jbc.M202343200. PMID 12011083.
- von Bahr S, Movin T, Papadogiannakis N, Pikuleva I, Rönnow P, Diczfalusy U, Björkhem I (July 2002). "Mechanism of accumulation of cholesterol and cholestanol in tendons and the role of sterol 27-hydroxylase (CYP27A1)". Arteriosclerosis, Thrombosis, and Vascular Biology. 22 (7): 1129–35. doi:10.1161/01.ATV.0000022600.61391.A5. PMID 12117727.
- Meir K, Kitsberg D, Alkalay I, Szafer F, Rosen H, Shpitzen S, Avi LB, Staels B, Fievet C, Meiner V, Björkhem I, Leitersdorf E (September 2002). "Human sterol 27-hydroxylase (CYP27) overexpressor transgenic mouse model. Evidence against 27-hydroxycholesterol as a critical regulator of cholesterol homeostasis". The Journal of Biological Chemistry. 277 (37): 34036–41. doi:10.1074/jbc.M201122200. PMID 12119285.
- Lee MJ, Huang YC, Sweeney MG, Wood NW, Reilly MM, Yip PK (September 2002). "Mutation of the sterol 27-hydroxylase gene ( CYP27A1) in a Taiwanese family with cerebrotendinous xanthomatosis". Journal of Neurology. 249 (9): 1311–2. doi:10.1007/s00415-002-0762-9. PMID 12242561. S2CID 32638499.
External links[]
- GeneReviews/NCBI/NIH/UW entry on Cerebrotendinous Xanthomatosis
- Cytochrome+P-450+CYP27A1 at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human CYP27A1 genome location and CYP27A1 gene details page in the UCSC Genome Browser.
- Genes on human chromosome 2
- Cytochrome P450