Ocean fertilization
Ocean fertilization or ocean nourishment is a type of climate engineering based on the purposeful introduction of nutrients to the upper ocean[2] to increase marine food production[3] and to remove carbon dioxide from the atmosphere. A number of techniques, including fertilization by iron, urea and phosphorus have been proposed. But research in the early 2020s suggested that it could only permanently sequester a small amount of carbon.[4]
History[]
John Martin, director of the Moss Landing Marine Laboratories, hypothesized that the low levels of phytoplankton in these regions are due to a lack of iron. In 1989 he tested this hypothesis (known as the Iron Hypothesis) by an experiment using samples of clean water from Antarctica.[5] Iron was added to some of these samples. After several days the phytoplankton in the samples with iron fertilization grew much more than in the untreated samples. This led Martin to speculate that increased iron concentrations in the oceans could partly explain past ice ages.[6]
IRONEX I[]
This experiment was followed by a larger field experiment (IRONEX I) where 445 kg of iron was added to a patch of ocean near the Galápagos Islands. The levels of phytoplankton increased three times in the experimental area.[7] The success of this experiment and others led to proposals to use this technique to remove carbon dioxide from the atmosphere.[8]
EisenEx[]
In 2000 and 2004, iron sulfate was discharged from the EisenEx. 10 to 20 percent of the resulting algal bloom died and sank to the sea floor.
Commercial projects[]
Planktos was a US company that abandoned its plans to conduct 6 iron fertilization cruises from 2007 to 2009, each of which would have dissolved up to 100 tons of iron over a 10,000 km2 area of ocean. Their ship Weatherbird II was refused entry to the port of Las Palmas in the Canary Islands where it was to take on provisions and scientific equipment.[9]
In 2007 commercial companies such as Climos and GreenSea Ventures and the Australian-based Ocean Nourishment Corporation, planned to engage in fertilization projects. These companies invited green co-sponsors to finance their activities in return for provision of carbon credits to offset investors' CO2 emissions.[10]
LOHAFEX[]
LOHAFEX was an experiment initiated by the German Federal Ministry of Research and carried out by the German Alfred Wegener Institute (AWI) in 2009 to study fertilization in the South Atlantic. India was also involved.[11]
As part of the experiment, the German research vessel Polarstern deposited 6 tons of ferrous sulfate in an area of 300 square kilometers. It was expected that the material would distribute through the upper 15 metres (49 ft) of water and trigger an algal bloom. A significant part of the carbon dioxide dissolved in sea water would then be bound by the emerging bloom and sink to the ocean floor.
The Federal Environment Ministry called for the experiment to halt, partly because environmentalists predicted damage to marine plants. Others predicted long-term effects that would not be detectable during short-term observation[12][unreliable source?] or that this would encourage large-scale ecosystem manipulation.[13][unreliable source?][14]
2012[]
A 2012 study deposited iron fertilizer in an eddy near Antarctica. The resulting algal bloom sent a significant amount of carbon into the deep ocean, where it was expected to remain for centuries to millennia. The eddy was chosen because it offered a largely self-contained test system.[15]
As of day 24, nutrients, including nitrogen, phosphorus and silicic acid that diatoms use to construct their shells, declined. Dissolved inorganic carbon concentrations were reduced below equilibrium with atmospheric CO
2. In surface water, particulate organic matter (algal remains) including silica and chlorophyll increased.[15]
After day 24, however, the particulate matter fell to between 100 metres (330 ft) to the ocean floor. Each iron atom converted at least 13,000 carbon atoms into algae. At least half of the organic matter sank below, 1,000 metres (3,300 ft).[15]
Haida Gwaii project[]
In July 2012, the Haida Salmon Restoration Corporation dispersed 100 short tons (91 t) of iron sulphate dust into the Pacific Ocean several hundred miles west of the islands of Haida Gwaii. The Old Massett Village Council financed the action as a salmon enhancement project with $2.5 million in village funds.[16] The concept was that the formerly iron-deficient waters would produce more phytoplankton that would in turn serve as a "pasture" to feed salmon. Then-CEO Russ George hoped to sell carbon offsets to recover the costs. The project was accompanied by charges of unscientific procedures and recklessness. George contended that 100 tons was negligible compared to what naturally enters the ocean.[17]
Some environmentalists called the dumping a "blatant violation" of two international moratoria.[16][18] George said that the Old Massett Village Council and its lawyers approved the effort and at least seven Canadian agencies were aware of it.[17]
According to George, the 2013 salmon runs increased from 50 million to 226 million fish.[19] However, many experts contend that changes in fishery stocks since 2012 cannot necessarily be attributed to the 2012 iron fertilization; many factors contribute to predictive models, and most data from the experiment are considered to be of questionable scientific value.[20]
On 15 July 2014, the data gathered during the project were made publicly available under the ODbL license.[21]
International reaction[]
In 2007 Working Group III of the United Nations Intergovernmental Panel on Climate Change examined ocean fertilization methods in its fourth assessment report and noted that the field-study estimates of the amount of carbon removed per ton of iron was probably over-estimated and that potential adverse effects had not been fully studied.[22]
In June 2007 the London Dumping Convention issued a statement of concern noting 'the potential for large scale ocean iron fertilization to have negative impacts on the marine environment and human health',.[23] but did not define 'large scale'. It is believed that the definition would include operations.[citation needed]
In 2008, the London Convention/London Protocol noted in resolution LC-LP.1 that knowledge on the effectiveness and potential environmental impacts of ocean fertilization was insufficient to justify activities other than research. This non-binding resolution stated that fertilization, other than research, "should be considered as contrary to the aims of the Convention and Protocol and do not currently qualify for any exemption from the definition of dumping".[24]
In May 2008, at the Convention on Biological Diversity, 191 nations called for a ban on ocean fertilization until scientists better understand the implications.[25]
In August 2018, Germany banned the sale of ocean seeding as carbon sequestration system[26] while the matter was under discussion at EU and levels.[27]
Rationale[]
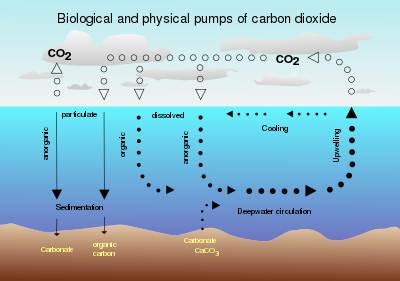
2 sequestration in the ocean
The marine food chain is based on photosynthesis by marine phytoplankton that combine carbon with inorganic nutrients to produce organic matter. Production is limited by the availability of nutrients, most commonly nitrogen or iron. Numerous experiments[28] have demonstrated how iron fertilization can increase phytoplankton productivity. Nitrogen is a limiting nutrient over much of the ocean and can be supplied from various sources, including fixation by cyanobacteria. Carbon-to-iron ratios in phytoplankton are much larger than carbon-to-nitrogen or carbon-to-phosphorus ratios, so iron has the highest potential for sequestration per unit mass added.
Oceanic carbon naturally cycles between the surface and the deep via two "pumps" of similar scale. The "solubility" pump is driven by ocean circulation and the solubility of CO2 in seawater. The "biological" pump is driven by phytoplankton and subsequent settling of detrital particles or dispersion of dissolved organic carbon. The former has increased as a result of increasing atmospheric CO2 concentration. This CO2 sink is estimated to be approximately 2 GtC yr−1.[29]
The global phytoplankton population fell about 40 percent between 1950 and 2008 or about 1 percent per year. The most notable declines took place in polar waters and in the tropics. The decline is attributed to sea surface temperature increases.[30] A separate study found that diatoms, the largest type of phytoplankton, declined more than 1 percent per year from 1998 to 2012, particularly in the North Pacific, North Indian and Equatorial Indian oceans. The decline appears to reduce pytoplankton's ability to sequester carbon in the deep ocean.[31]
Fertilization offers the prospect of both reducing the concentration of atmospheric greenhouse gases with the aim of slowing climate change and at the same time increasing fish stocks via increasing primary production. The reduction reduces the ocean's rate of carbon sequestration in the deep ocean.
Each area of the ocean has a base sequestration rate on some timescale, e.g., annual. Fertilization must increase that rate, but must do so on a scale beyond the natural scale. Otherwise, fertilization changes the timing, but not the total amount sequestered. However, accelerated timing may have beneficial effects for primary production separate from those from sequestration.[29]
Biomass production inherently depletes all resources (save for sun and water). Either they must all be subject to fertilization or sequestration will eventually be limited by the one mostly slowly replenished (after some number of cycles) unless the ultimate limiting resource is sunlight and/or surface area. Generally, phosphate is the ultimate limiting nutrient. As oceanic phosphorus is depleted (via sequestration) it would have to be included in the fertilization cocktail supplied from terrestrial sources.[29]
Approaches[]
"Ocean fertilisation options are only worthwhile if sustained on a millennial timescale and phosphorus addition may have greater long-term potential than iron or nitrogen fertilisation."[32] Phytoplankton require a variety of nutrients. These include macronutrients such as nitrate and phosphate (in relatively high concentrations) and micronutrients such as iron and zinc (in much smaller quantities). Nutrient requirements vary across phylogenetic groups (e.g., diatoms require silicon) but may not individually limit total biomass production. Co-limitation (among multiple nutrients) may also mean that one nutrient can partially compensate for a shortage of another. Silicon does not affect total production, but can change the timing and community structure with follow-on effects on remineralization times and subsequent mesopelagic.nutrient vertical distribution.[29]
High-nutrient, low-chlorophyll (LNLC) waters occupy the oceans' subtropical gyre systems, approximately 40 per cent of the surface, where wind-driven downwelling and a strong thermocline impede nutrient resupply from deeper water. Nitrogen fixation by cyanobacteria provides a major source of N. In effect, it ultimately prevents the ocean from losing the N required for photosynthesis. Phosphorus has no substantial supply route, making it the ultimate limiting macronutrient. The sources that fuel primary production are deep water stocks and runoff or dust-based.[29]
Iron[]
Approximately 25 per cent of the ocean surface has ample macronutrients, with little plant biomass (as defined by chlorophyll). The production in these high-nutrient low-chlorophyll (HNLC) waters is primarily limited by micronutrients especially iron.[29] The cost of distributing iron over large ocean areas is large compared with the expected value of carbon credits.[33]
Phosphorus[]
In the very long term, phosphorus "is often considered to be the ultimate limiting macronutrient in marine ecosystems"[34] and has a slow natural cycle. Where phosphate is the limiting nutrient in the photic zone, addition of phosphate is expected to increase primary phytoplankton production. This technique can give 0.83W/m2 of globally averaged negative forcing,[32] which is sufficient to reverse the warming effect of about half the current levels of anthropogenic CO
2 emissions. One water-soluble fertilizer is diammonium phosphate (DAP), (NH
4)
2HPO
4, that as of 2008 had a market price of 1700/tonne−1 of phosphorus. Using that price and the C : P Redfield ratio of 106 : 1 produces a sequestration cost (excluding preparation and injection costs) of some $45 /tonne of carbon (2008), substantially less than the trading price for carbon emissions.[29]
Nitrogen[]
This technique (proposed by Ian Jones) proposes to fertilize the ocean with urea, a nitrogen rich substance, to encourage phytoplankton growth.[35] This has also been considered by Karl.[36] Concentrations of macronutrients per area of ocean surface would be similar to large natural upwellings. Once exported from the surface, the carbon remains sequestered for a long time.[37]
An Australian company, Ocean Nourishment Corporation (ONC), planned to inject hundreds of tonnes of urea into the ocean, in order to boost the growth of CO
2-absorbing phytoplankton, as a way to combat climate change. In 2007, Sydney-based ONC completed an experiment involving one tonne of nitrogen in the Sulu Sea off the Philippines.[38]
Macronutrient nourishment can give 0.38W/m2 of globally averaged negative forcing,[32] which is sufficient to reverse the warming effect of current levels of around a quarter of anthropogenic CO
2 emissions.
The Ocean Nourishment Corporation claimed, "One Ocean Nourishment plant will remove approximately 5–8 million tonnes of CO2 from the atmosphere for each year of operation, equivalent to offsetting annual emissions from a typical 1200 MW coal-fired power station or the short-term sequestration from one million hectares of new growth forest".[39]
The two dominant costs are manufacturing the nitrogen and nutrient delivery.[40]
Pelagic pumping[]
Local wave power could be used to pump nutrient-rich water from hundred- metre-plus depths to the euphotic zone. However, deep water concentrations of dissolved CO2 could be returned to the atmosphere.[29]
The supply of DIC in upwelled water is generally sufficient for photosynthesis permitted by upwelled nutrients, without requiring atmospheric CO2. Second-order effects include how the composition of upwelled water differs from that of settling particles. More nitrogen than carbon is remineralized from sinking organic material. Upwelling of this water allows more carbon to sink than that in the upwelled water, which would make room for at least some atmospheric CO2 to be absorbed. the magnitude of this difference is unclear. No comprehensive studies have yet resolved this question. Preliminary calculations using upper limit assumptions indicate a low value. 1,000 square kilometres (390 sq mi) could sequester 1 gigatonne/year.[29]
Sequestration thus depends on the upward flux and the rate of lateral surface mixing of the surface water with denser pumped water.[29]
Volcanic ash[]
Volcanic ash adds nutrients to the surface ocean. This is most apparent in nutrient-limited areas. Research on the effects of anthropogenic and aeolian iron addition to the ocean surface suggests that nutrient-limited areas benefit most from a combination of nutrients provided by anthropogenic, eolian and volcanic deposition.[41] Some oceanic areas are comparably limited in more than one nutrient, so fertilization regimes that includes all limited nutrients is more likely to succeed. Volcanic ash supplies multiple nutrients to the system, but excess metal ions can be harmful. The positive impacts of volcanic ash deposition are potentially outweighed by their potential to do harm.[citation needed]
Clear evidence documents that ash can be as much as 45 percent by weight in some deep marine sediments.[42][43] In the Pacific Ocean estimates claim that (on a millennial-scale) the atmospheric deposition of air-fall volcanic ash was as high as the deposition of desert dust.[44] This indicates the potential of volcanic ash as a significant iron source.
In August 2008 the Kasatochi volcanic eruption in the Aleutian Islands, Alaska, deposited ash in the nutrient-limited northeast Pacific. This ash (including iron) resulted in one of the largest phytoplankton blooms observed in the subarctic.[45][46] Fisheries scientists in Canada linked increased oceanic productivity from the volcanic iron to subsequent record returns of salmon in the Fraser River two years later[47]
Monitored nutrients[]
The approach advocated by Ocean Nutrition Corporation is to limit the distribution of added nutrients to allow phytoplankton concentrations to rise only to the values seen in upwelling regions (5-10 mg Chl /m3). Maintaining healthy phytoplankton levels is claimed to avoid harmful algal blooms and oxygen depletion. Chlorophyll concentration is an easily measured proxy for phytoplankton concentration. The company stated that values of approximately 4 mg Chl/m3 meet this requirement.[48]SS
Complications[]
While manipulation of the land ecosystem in support of agriculture for the benefit of humans has long been accepted (despite its side effects), directly enhancing ocean productivity has not. Among the reasons are:
Outright opposition[]
According to Lisa Speer of the Natural Resources Defense Council, "There is a limited amount of money, of time, that we have to deal with this problem....The worst possible thing we could do for climate change technologies would be to invest in something that doesn't work and that has big impacts that we don't anticipate."[49]
In 2009 Aaron Strong, Sallie Chisholm, Charles Miller and John Cullen opined in Nature "...fertilizing the oceans with iron to stimulate phytoplankton blooms, absorb carbon dioxide from the atmosphere and export carbon to the deep sea — should be abandoned."[50]
Efficiency[]
Algal cell chemical composition is often assumed to respect a ratio where atoms are 106 carbon: 16 nitrogen: 1 phosphorus (Redfield ratio[51]): 0.0001 iron. In other words, each atom of iron helps capture 1,060,000 atoms of carbon, while one nitrogen atom only 6.[52]
In large areas of the ocean, such organic growth (and hence nitrogen fixation) is thought to be limited by the lack of iron rather than nitrogen, although direct measures are hard.[51]
On the other hand, experimental iron fertilisation in HNLC regions has been supplied with excess iron which cannot be utilized before it is scavenged. Thus the organic material produced was much less than if the ratio of nutrients above were achieved. Only a fraction of the available nitrogen (because of iron scavenging) is drawn down. In culture bottle studies of oligotrophic water, adding nitrogen and phosphorus can draw down considerably more nitrogen per dosing. The export production is only a small percentage of the new primary production and in the case of iron fertilization, iron scavenging means that regenerative production is small. With macronutrient fertilisation, regenerative production is expected to be large and supportive of larger total export. Other losses can also reduce efficiency.[53]
In addition, the efficiency of carbon sequestration through ocean fertilisation is heavily influenced by factors such as changes in stoichiometric ratios and gas exchange make accurately predicting the effectiveness of ocean feralization projects.[54]
Side effects[]
According to Gnadesikan and Marinou, 2008, Beyond biological impacts, evidences suggests that plankton blooms can affect the physical properties of surface waters simply by absorbing light and heat from the sun. Watson added that if fertilization is done in shallow coastal waters, a dense layer of phytoplankton clouding the top 30 metres or so of the ocean could hinder corals, kelps or other deeper sea life from carrying out photosynthesis (Watson et al. 2008). In addition, as the bloom declines, nitrous oxide is released, potentially counteracting the effects from the sequestering of carbon.[55]
Algal blooms[]
Toxic algal blooms are common in coastal areas. Fertilization could trigger such blooms. Chronic fertilization could risk the creation of dead zones, such as the one in the Gulf of Mexico.[15]
Impact on fisheries[]
Adding urea to the ocean can cause phytoplankton blooms that serve as a food source for zooplankton and in turn feed for fish. This may increase fish catches.[56] However, if cyanobacteria and dinoflagellates dominate phytoplankton assemblages that are considered poor quality food for fish then the increase in fish quantity may not be large.[57] Some evidence links iron fertilization from volcanic eruptions to increased fisheries production.[47][45] Other nutrients would be metabolized along with the added nutrient(s), reducing their presence in fertilized waters.[49]
Krill populations have declined dramatically since whaling began.[15] Sperm whales transport iron from the deep ocean to the surface during prey consumption and defecation. Sperm whales have been shown to increase the levels of primary production and carbon export to the deep ocean by depositing iron-rich faeces into surface waters of the Southern Ocean. The faeces causes phytoplankton to grow and take up carbon. The phytoplankton nourish krill. Reducing the abundance of sperm whales in the Southern Ocean, whaling resulted in an extra 2 million tonnes of carbon remaining in the atmosphere each year.[58]
Ecosystem disruption[]
Many locations, such as the Tubbataha Reef in the Sulu Sea, support high marine biodiversity.[59] Nitrogen or other nutrient loading in coral reef areas can lead to community shifts towards algal overgrowth of corals and ecosystem disruption, implying that fertilization must be restricted to areas in which vulnerable populations are not put at risk.[60]
As the phytoplankton descend the water column, they decay, consuming oxygen and producing greenhouse gases methane and nitrous oxide. Plankton-rich surface waters could warm the surface layer, affecting circulation patterns.[49]
Cloud formation[]
Many phytoplankton species release dimethyl sulfide (DMS), which escapes into the atmosphere where it forms sulfate aerosols and encourages cloud formation, which could reduce warming.[49] However, substantial increases in DMS could reduce global rainfall, according to global climate model simulations, while halving temperature increases as of 2100.[61][62]
International Law[]
International law presents some dilemmas for ocean fertilization.[citation needed] The United Nations Framework Convention on Climate Change (UNFCCC 1992) has accepted mitigation actions.[citation needed] However, the UNFCCC and its revisions recognise only forestation and reforestation projects as carbon sinks.[citation needed]
Law of the sea[]
According to United Nations Convention on the Law of the Sea (LOSC 1982), all states are obliged to take all measures necessary to prevent, reduce and control pollution of the marine environment, to prohibit the transfer of damage or hazards from one area to another and to prohibit the transformation of one type pollution to another. How this relates to fertilization is undetermined.[63]
Solar radiation management[]
Fertilization may create sulfate aerosols that reflect sunlight, modifying the Earth's albedo, creating a cooling effect that reduces some of the effects of climate change. Enhancing the natural sulfur cycle in the Southern Ocean[64] by fertilizing with iron in order to enhance dimethyl sulfide production and cloud reflectivity may achieve this.[65][66]
See also[]
- Carbon dioxide sink
- Climate engineering
- Planetary engineering
- Soil fertilization
References[]
- ^ NASA Goddard Multimedia Accessed June 2012
- ^ Matear, R. J. & B. Elliott (2004). "Enhancement of oceanic uptake of anthropogenic CO2 by macronutrient fertilization". J. Geophys. Res. 109 (C4): C04001. Bibcode:2004JGRC..10904001M. doi:10.1029/2000JC000321.
- ^ Jones, I.S.F. & Young, H.E. (1997). "Engineering a large sustainable world fishery". Environmental Conservation. 24 (2): 99–104. doi:10.1017/S0376892997000167.
- ^ "Cloud spraying and hurricane slaying: how ocean geoengineering became the frontier of the climate crisis". the Guardian. 23 June 2021. Retrieved 23 June 2021.
- ^ "The Iron Hypothesis". www.homepages.ed.ac.uk. Retrieved 26 July 2020.
- ^ John Weier (10 July 2001). "The Iron Hypothesis". John Martin (1935–1993). Retrieved 27 August 2012.
- ^ John Weier (10 July 2001). "Following the vision". John Martin (1935–1993). Retrieved 27 August 2012.
- ^ Richtel, Matt (1 May 2007). "Recruiting Plankton to Fight Global Warming". The New York Times. Retrieved 3 June 2017.
- ^ "Planktos Shareholder Update". Business wire. 19 December 2007. Archived from the original on 25 June 2008.
- ^ Salleh, Anna (2007). "Urea 'climate solution' may backfire". ABC Science Online. Archived from the original on 18 November 2008.
- ^ "Alfred-Wegener-Institut für Polar- und Meeresforschung (AWI) ANT-XXV/3". Archived from the original on 8 October 2012. Retrieved 9 August 2012.
- ^ "LOHAFEX über sich selbst". 14 January 2009. Retrieved 9 August 2012.
- ^ Helfrich, Silke (12 January 2009). "Polarsternreise zur Manipulation der Erde". CommonsBlog. Retrieved 3 June 2017.
- ^ John, Paull (2009). "Geo-Engineering in the Southern Ocean". Journal of Bio-Dynamics Tasmania (93). Retrieved 3 June 2017.
- ^ Jump up to: a b c d e "Could Fertilizing the Oceans Reduce Global Warming?". Live Science. Retrieved 2 June 2017.
- ^ Jump up to: a b Lucas, Martin (15 October 2012). "World's biggest geoengineering experiment 'violates' UN rules". The Guardian. Retrieved 17 October 2012.
- ^ Jump up to: a b Fountain, Henry (18 October 2012). "A Rogue Climate Experiment Outrages Scientists". The New York Times. Retrieved 18 October 2012.
- ^ "Environment Canada launches probe into massive iron sulfate dump off Haida Gwaii coast". APTN National News. 16 October 2012. Retrieved 17 October 2012.
- ^ Zubrin, Robert (22 April 2014). "The Pacific's Salmon Are Back — Thank Human Ingenuity". Nationalreview.com. Retrieved 23 April 2014.
- ^ "Controversial Haida Gwaii ocean fertilizing experiment pitched to Chile". CBC News. Retrieved 9 November 2017.
- ^ "WELCOME TO THE OCB OCEAN FERTILIZATION WEBSITE".
- ^ B. Metz; O.R. Davidson; P.R. Bosch; R. Dave; L.A. Meyer, eds. (2007). "11.2.2". Climate Change 2007: Working Group III: Mitigation of Climate Change. Cambridge University Press. Retrieved 27 August 2012.
- ^ "Scientific Groups cautious over iron fertilization of the oceans to sequester CO2". Retrieved 27 August 2012.
- ^ RESOLUTION LC-LP.1 (2008) ON THE REGULATION OF OCEAN FERTILIZATION (PDF). London Dumping Convention. 31 October 2008. Retrieved 9 August 2012.
- ^ Tollefson, Jeff (5 June 2008). "UN decision puts brakes on ocean fertilization". Nature. 453 (7196): 704. doi:10.1038/453704b. ISSN 0028-0836. PMID 18528354.
- ^ 2-paris-agreement-carbon-europe-mulls-stripping-from-the-skies/ "Europe mulls stripping carbon from the skies". POLITICO. 9 August 2018. Retrieved 16 August 2018.
In early August, Germany decided that ocean seeding will only be allowed for research purposes and under strict conditions.
- ^ Chestney, Nina. "CO2 removal 'no silver bullet' to fighting climate change-scientists". Reuters. Retrieved 16 August 2018.[dead link]
- ^ Coale KH, Johnson KS, Fitzwater SE, et al. (October 1996). "A massive phytoplankton bloom induced by an ecosystem-scale iron fertilization experiment in the equatorial Pacific Ocean". Nature. 383 (6600): 495–501. Bibcode:1996Natur.383..495C. doi:10.1038/383495a0. PMID 18680864. S2CID 41323790.
- ^ Jump up to: a b c d e f g h i j Lampitt, R. S.; Achterberg, E. P.; Anderson, T. R.; Hughes, J. A.; Iglesias-Rodriguez, M. D.; Kelly-Gerreyn, B. A.; Lucas, M.; Popova, E. E.; Sanders, R. (13 November 2008). "Ocean fertilization: a potential means of geoengineering?". Philosophical Transactions of the Royal Society of London A: Mathematical, Physical and Engineering Sciences. 366 (1882): 3919–3945. Bibcode:2008RSPTA.366.3919L. doi:10.1098/rsta.2008.0139. ISSN 1364-503X. PMID 18757282.
- ^ Morello, Lauren (29 July 2010). "Phytoplankton Population Drops 40 Percent Since 1950". Scientific American. Retrieved 19 July 2018.
- ^ Morrow, Ashley (22 September 2015). "Study Shows Phytoplankton Declines in Northern Hemisphere". NASA. Retrieved 19 July 2018.
- ^ Jump up to: a b c Lenton, T. M.; Vaughan, N. E. (2009). "The radiative forcing potential of different climate geoengineering options". Atmos. Chem. Phys. 9 (15): 5539–5561. Bibcode:2009ACP.....9.5539L. doi:10.5194/acp-9-5539-2009.
- ^ Harrison, Daniel P. (2013). "A method for estimating the cost to sequester carbon dioxide by delivering iron to the ocean". International Journal of Global Warming. 5 (3): 231. doi:10.1504/ijgw.2013.055360.
- ^ Paytan, Adina; McLaughlin, Karen (2007). "The Oceanic Phosphorus Cycle" (PDF). Chemical Reviews. 107 (2): 563–576. CiteSeerX 10.1.1.417.3956. doi:10.1021/cr0503613. ISSN 1520-6890. PMID 17256993. S2CID 1872341. Archived from the original (PDF) on 16 August 2018.
- ^ Jones, Ian S. F. (1996). "Enhanced Carbon Dioxide Uptake by the World's Oceans". Energy Conversion & Management. 37 (6–8): 1049–1052. doi:10.1016/0196-8904(95)00296-0. Note typo in paper Fig. 1.
- ^ Karl, D. M.; Letelier, R. (2008). "Nitrogen fixation-enhanced carbon sequestration in low nitrate, low chlorophyll seascapes". Mar. Ecol. Prog. Ser. 364: 257–268. Bibcode:2008MEPS..364..257K. doi:10.3354/meps07547.
- ^ Jones, Ian S F; Harrison, D P (4 June 2013). Richmond, Amos; Hu, Qiang (eds.). Handbook of Microalgal Culture: Applied Phycology and Biotechnology (2 ed.). Wiley. ISBN 978-0-470-67389-8.
- ^ Anna Salleh (9 November 2007). "Urea 'climate solution' may backfire". ABC Science: In Depth. Australian Broadcasting Commission.
- ^ "Technology". Ocean Nourishment Corporation. Archived from the original on 19 October 2012. Retrieved 27 August 2012.
- ^ Ian S.F. Jones (10 November 2014). "The cost of carbon management using ocean nourishment". International Journal of Climate Change Strategies and Management. 6 (4): 391–400. doi:10.1108/ijccsm-11-2012-0063. ISSN 1756-8692.
- ^ Duggen, Svend; Croot, Peter; Schacht, Ulrike; Hoffmann, Linn (2007). "Subduction zone volcanic ash can fertilize the surface ocean and stimulate phytoplankton growth: Evidence from biogeochemical experiments and satellite data". Geophysical Research Letters. 34. Bibcode:2007GeoRL..3401612D. doi:10.1029/2006GL027522. Retrieved 27 August 2012.
- ^ Peters, J.L.; Murray, R.W.; Sparks, J.W; Coleman, D.S. (2000). "Terrigenous matter and dispersed ash in sediment from the Caribbean Sea; results from Leg 165". Proceedings of the Ocean Drilling Program, Scientific Results. Proceedings of the Ocean Drilling Program. 165: 115–124. doi:10.2973/odp.proc.sr.165.003.2000.
- ^ Scudder, Rachel P.; Murray, Richard W.; Plank, Terry (15 July 2009). "Dispersed ash in deeply buried sediment from the northwest Pacific Ocean: An example from the Izu–Bonin arc (ODP Site 1149)". Earth and Planetary Science Letters. 284 (3–4): 639–648. Bibcode:2009E&PSL.284..639S. doi:10.1016/j.epsl.2009.05.037.
- ^ Olgun, Nazlı; Duggen, Svend; Croot, Peter Leslie; Delmelle, Pierre; Dietze, Heiner; Schacht, Ulrike; Óskarsson, Niels; Siebe, Claus; Auer, Andreas (1 December 2011). "Surface ocean iron fertilization: The role of airborne volcanic ash from subduction zone and hot spot volcanoes and related iron fluxes into the Pacific Ocean" (PDF). Global Biogeochemical Cycles. 25 (4): GB4001. Bibcode:2011GBioC..25.4001O. doi:10.1029/2009gb003761. ISSN 1944-9224.
- ^ Jump up to: a b Olgun, N; Duggen, S; Langmann, B; Hort, M; Waythomas, CF; Hoffmann, L; Croot, P (15 August 2013). "Geochemical evidence of oceanic iron fertilization by the Kasatochi volcanic eruption in 2008 and the potential impacts on Pacific sockeye salmon" (PDF). Marine Ecology Progress Series. 488: 81–88. Bibcode:2013MEPS..488...81O. doi:10.3354/meps10403. ISSN 0171-8630.
- ^ Hemme, R; et al. (2010). "Volcanic ash fuels anomalous plankton bloom in subarctic northeast Pacific". Geophysical Research Letters. 37 (19): L19604. Bibcode:2010GeoRL..3719604H. doi:10.1029/2010GL044629. Retrieved 27 August 2012.
- ^ Jump up to: a b Parsons, Timothy R.; Whitney, Frank A. (1 September 2012). "Did volcanic ash from Mt. Kasatoshi in 2008 contribute to a phenomenal increase in Fraser River sockeye salmon (Oncorhynchus nerka) in 2010?". Fisheries Oceanography. 21 (5): 374–377. doi:10.1111/j.1365-2419.2012.00630.x. ISSN 1365-2419.
- ^ "Ocean Solutions". ocean nourishment. Retrieved 14 August 2021.
- ^ Jump up to: a b c d "Fertilizing the Ocean with Iron". Oceanus Magazine. Retrieved 1 June 2017.
- ^ Strong, Aaron; Chisholm, Sallie; Miller, Charles; Cullen, John (2009). "Ocean fertilization: time to move on". Nature. 461 (7262): 347–348. Bibcode:2009Natur.461..347S. doi:10.1038/461347a. PMID 19759603. S2CID 205049552.
- ^ Jump up to: a b Falkowski, Paul G. (9 February 2000). "Rationalizing elemental ratios in unicellular algae" (PDF). Journal of Phycology. 36 (1): 3–6. doi:10.1046/j.1529-8817.2000.99161.x. ISSN 1529-8817. S2CID 2185706.
- ^ P.M. Glibert et al., 2008. Ocean urea fertilization for carbon credits poses high ecological risks. Marine Pollution Bulletin, 56(2008): 1049–1056.
- ^ Lawrence, Martin W. (2014). "Efficiency of carbon sequestration by added reactive nitrogen in ocean fertilisation". International Journal of Global Warming. 6 (1): 15. doi:10.1504/ijgw.2014.058754.
- ^ Gnanadesikan, Anand; Marinov, Irina (29 July 2008). "Export is not enough: nutrient cycling and carbon sequestration". Marine Ecology Progress Series. 364: 289–294. doi:10.3354/meps07550. ISSN 0171-8630.
- ^ Law, C. S. (29 July 2008). "Predicting and monitoring the effects of large-scale ocean iron fertilization on marine trace gas emissions". Marine Ecology Progress Series. 364: 283–288. doi:10.3354/meps07549. ISSN 0171-8630.
- ^ Jones, I; Renilson, M (2011). "Using Ocean Nourishment to increase fishing effectiveness". The Journal of Ocean Technology – One Voice for the World's Oceans Community (6): 30–37. Retrieved 3 June 2017.
- ^ Glibert, P M.; et al. (2008). "Ocean urea fertilization for carbon credits poses high ecological risks" (PDF). Marine Pollution Bulletin. 56 (6): 1049–1056. doi:10.1016/j.marpolbul.2008.03.010. PMC 5373553. PMID 18439628. Retrieved 27 August 2012.
- ^ Lavery, Trish J.; Roudnew, Ben; Gill, Peter; Seymour, Justin; Seuront, Laurent; Johnson, Genevieve; Mitchell, James G.; Smetacek, Victor (22 November 2010). "Iron defecation by sperm whales stimulates carbon export in the Southern Ocean". Proceedings of the Royal Society of London B: Biological Sciences. 277 (1699): 3527–3531. doi:10.1098/rspb.2010.0863. ISSN 0962-8452. PMC 2982231. PMID 20554546.
- ^ Mission, G., 1999. WWF's marine police: saving Sulu Sea
- ^ Smith, S.V.; Kimmerer, W.J.; Laws, E.A.; Brock, R.E.; Walsh, T.W. (1981). "Kaneohe Bay sewage diversion experiment: perspectives on ecosystem responses to nutritional perturbation". Pacific Science. 35: 279–395. Retrieved 4 June 2017.
- ^ Grandey, B. S.; Wang, C. (21 August 2015). "Enhanced marine sulphur emissions offset global warming and impact rainfall". Scientific Reports. 5 (1): 13055. Bibcode:2015NatSR...513055G. doi:10.1038/srep13055. ISSN 2045-2322. PMC 4543957. PMID 26293204.
- ^ "Fertilize the ocean, cool the planet?". MIT News. Retrieved 2 June 2017.
- ^ Mayo-Ramsay, J P, Climate Change Mitigation Strategies: Ocean Fertilisation, The Argument For & Against (2012)
- ^ Wingenter, Oliver W.; Elliot, Scott M.; Blake, Donald R. (1 November 2007). "New Directions: Enhancing the natural sulfur cycle to slow global warming". Atmospheric Environment. 41 (34): 7373–7375. Bibcode:2007AtmEn..41.7373W. doi:10.1016/j.atmosenv.2007.07.021.
- ^ "Slowing Global Warming by Enhancing the Natural Sulfur Cycle". Archived from the original on 8 July 2011.
- ^ Coale, K. H.; Johnson, K. S.; Buesseler, K.; Sofex Group (2002). "SOFeX: Southern Ocean Iron Experiments. Overview and Experimental Design". AGU Fall Meeting Abstracts. 2002: OS22D–01. Bibcode:2002AGUFMOS22D..01C.
External links[]
- Williamson, Phillip; Wallace, Douglas W. R.; Law, Cliff S.; Boyd, Philip W.; Collos, Yves; Croot, Peter; Denman, Ken; Riebesell, Ulf; Takeda, Shigenobu (1 November 2012). "Ocean fertilization for geoengineering: A review of effectiveness, environmental impacts and emerging governance". Process Safety and Environmental Protection. 90 (6): 475–488. doi:10.1016/j.psep.2012.10.007. ISSN 0957-5820.
- Dean, Jennie (2009). "Iron Fertilization: A Scientific Review with International Policy Recommendations" (PDF). Retrieved 4 June 2017.
- "Ocean fertilization" (PDF). geoengineeringmonitor.org. January 2021.
- Aquatic ecology
- Fisheries science
- Planetary engineering
- Oceanographical terminology
- Climate engineering


