Clogestone acetate
 | |
| Clinical data | |
|---|---|
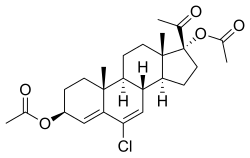
| Other names | Chlormadinol acetate; AY-11440; 3β,17α-Diacetoxy-6-chloropregna-4,6-diene-20-one |
| Drug class | Progestogen; Progestogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H33ClO5 |
| Molar mass | 448.98 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clogestone acetate (USAN) (developmental code name AY-11440), also known as chlormadinol acetate or as 3β,17α-diacetoxy-6-chloropregna-4,6-diene-20-one,[1] is a steroidal progestin which was investigated as a progestin-only contraceptive and postcoital contraceptive but was never marketed.[2][3][4][5] It is the diacetate ester of clogestone, which, similarly was never marketed.[2] Clogestone acetate produces chlormadinone acetate as an active metabolite.[6][7]
See also[]
References[]
- ^ Gerald Litwack (2 December 2012). Biochemical Actions of Hormones. Elsevier. pp. 323–. ISBN 978-0-323-15189-4.
- ^ a b J. Elks (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 297–. ISBN 978-1-4757-2085-3.
- ^ D F Hawkins; M G Elder (22 October 2013). Human Fertility Control: Theory and Practice. Elsevier Science. pp. 3–. ISBN 978-1-4831-6361-1.
- ^ JUCKER (8 March 2013). Progress in Drug Research / Fortschritte der Arzneimittelforschung / Progrès des rechersches pharmaceutiques. Birkhäuser. pp. 321–. ISBN 978-3-0348-7098-6.
- ^ Hinselmann M, Jürgensen O, Hasselblatt I, Otten U, Prinz W, Taubert HD (1970). "[Clogestone acetate, a new orally effective progestagen]". Arch Gynakol (in German). 209 (2): 136–48. doi:10.1007/BF00668180. PMID 5537778.
- ^ Stern, Michael D.; Givner, Morris L. (1975). "Measurement of Serum Clogestone Acetate (Ay-11, 440) by a Radioreceptor Assay: Practical Approach to the Quantitative Determination of Synthetic Progestins". The Journal of Clinical Endocrinology & Metabolism. 40 (4): 728–731. doi:10.1210/jcem-40-4-728. ISSN 0021-972X. PMID 1127082.
- ^ Nuclear Science Abstracts. Oak Ridge Directed Operations, Technical Information Division. July 1975. p. 127.
Categories:
- Abandoned drugs
- Acetate esters
- Organochlorides
- Pregnanes
- Progestogen esters
- Progestogens
- Genito-urinary system drug stubs
- Steroid stubs