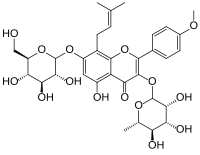
Icariin
| |
| Names | |
|---|---|
| IUPAC name
7-(β-D-Glucopyranosyloxy)-5-hydroxy-4′-methoxy-8-(3-methylbut-2-en-1-yl)-3-(α-L-rhamnopyranosyloxy)flavone
| |
| Preferred IUPAC name
5-Hydroxy-2-(4-methoxyphenyl)-8-(3-methylbut-2-en-1-yl)-7-{[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-3-{[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxy}-4H-1-benzopyran-4-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.107.649 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C33H40O15 | |
| Molar mass | 676.668 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Icariin is a chemical compound classified as a prenylated flavonol glycoside, a type of flavonoid. It is the 8-prenyl derivative of kaempferol 3,7-O-diglucoside. The compound has been isolated from several species of plant belonging to the genus Epimedium which are commonly known as horny goat weed, Yin Yang Huo,[1] and Herba epimedii.[2] Extracts from these plants are reputed to produce aphrodisiac effects, and are used in traditional Chinese medicine to enhance erectile function.[3] However, clinical trial data are lacking to support these claims.[4]
Research[]
There is one published human trial. In this small, no-control trial with subjects with bipolar disorder, depression and alcohol consumption were decreased.[5] It is thought that icariin is an active component of Epimedium extracts, as it has been shown to share several mechanisms of action with compounds used in Western medicine to treat impotence. In particular, icariin is a weak PDE5 inhibitor in vitro[6][7][8] and enhances the production of nitric oxide,[9] as well as mimicking the effects of testosterone in rats.[10] In vitro evidence suggests it is a weak antioxidant.[11][12] It has antidepressant-like effects in mice.[13][14][15] It also has neuroprotective effects in rats.[16][17] It has also been shown to return the gut microbiota of aged mice to a youthful profile.[18]
References[]
- ^ Liu JJ, Li SP, Wang YT (2006). "Optimization for quantitative determination of four flavonoids in Epimedium by capillary zone electrophoresis coupled with diode array detection using central composite design". J Chromatogr A. 1103 (2): 344–349. doi:10.1016/j.chroma.2005.11.036. PMID 16337210.
- ^ Cai WJ, Huang JH, Zhang SQ, Wu B, Kapahi P, Zhang XM, Shen ZY (2011). Blagosklonny MV (ed.). "Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans". PLOS ONE. 6 (12): e28835. Bibcode:2011PLoSO...628835C. doi:10.1371/journal.pone.0028835. PMC 3244416. PMID 22216122.
- ^ Makarova MN, Pozharitskaya ON, Shikov AN, Tesakova SV, Makarov VG, Tikhonov VP (2007). "Effect of lipid-based suspension of Epimedium koreanum Nakai extract on sexual behavior in rats". J Ethnopharmacol. 114 (3): 412–416. doi:10.1016/j.jep.2007.08.021. PMID 17890032.
- ^ "Horny Goat Weed". Drugs.com. August 5, 2019. Retrieved November 7, 2019.
- ^ Xiao H, Wignall N, Brown ES (2016). "An open-label pilot study of icariin for co-morbid bipolar and alcohol use disorder". Am J Drug Alcohol Abuse. 42 (2): 162–167. doi:10.3109/00952990.2015.1114118. PMID 26809351. S2CID 207429144.
- ^ Jiang Z, Hu B, Wang J, et al. (2006). "Effect of icariin on cyclic GMP levels and on the mRNA expression of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in penile cavernosum". 26 (4): 460–462. doi:10.1007/s11596-006-0421-y. PMID 17120748. S2CID 39841158.
- ^ Ning H, Xin ZC, Lin G, Banie L, Lue TF, Lin CS (2006). "Effects of icariin on phosphodiesterase-5 activity in vitro and cyclic guanosine monophosphate level in cavernous smooth muscle cells". Urology. 68 (6): 1350–4. doi:10.1016/j.urology.2006.09.031. PMID 17169663.
- ^ Dell'Agli M, Galli GV, Dal Cero E, et al. (2008). "Potent inhibition of human phosphodiesterase-5 by icariin derivatives". J. Nat. Prod. 71 (9): 1513–1517. doi:10.1021/np800049y. PMID 18778098.
- ^ Xu HB, Huang ZQ (2007). "Icariin enhances endothelial nitric-oxide synthase expression on human endothelial cells in vitro". Vascul. Pharmacol. 47 (1): 18–24. doi:10.1016/j.vph.2007.03.002. PMID 17499557.
- ^ Zhang ZB, Yang QT (2006). "The testosterone mimetic properties of icariin". Asian J. Androl. 8 (5): 601–5. doi:10.1111/j.1745-7262.2006.00197.x. PMID 16751992.
- ^ Xie J, Sun W, Duan K, Zhang Y (2007). "Chemical constituents of roots of Epimedium wushanense and evaluation of their biological activities". Nat. Prod. Res. 21 (7): 600–605. doi:10.1080/14786410701369680. PMID 17613817. S2CID 43529526.
- ^ Zhao F, Tang YZ, Liu ZQ (2007). "Protective effect of icariin on DNA against radical-induced oxidative damage". J. Pharm. Pharmacol. 59 (12): 1729–1732. doi:10.1211/jpp.59.12.0016. PMID 18053336. S2CID 45808790.
- ^ Pan Y, Kong L, Xia X, Zhang W, Xia Z, Jiang F (2005). "Antidepressant-like effect of icariin and its possible mechanism in mice". Pharmacol. Biochem. Behav. 82 (4): 686–694. doi:10.1016/j.pbb.2005.11.010. PMID 16380159. S2CID 44465190.
- ^ Pan Y, Zhang WY, Xia X, Kong LD (2006). "Effects of icariin on hypothalamic-pituitary-adrenal axis action and cytokine levels in stressed Sprague-Dawley rats". Biol. Pharm. Bull. 29 (12): 2399–2403. doi:10.1248/bpb.29.2399. PMID 17142971.
- ^ Pan Y, Kong LD, Li YC, Xia X, Kung HF, Jiang FX (2007). "Icariin from Epimedium brevicornum attenuates chronic mild stress-induced behavioral and neuroendocrinological alterations in male Wistar rats". Pharmacol. Biochem. Behav. 87 (1): 130–140. doi:10.1016/j.pbb.2007.04.009. PMID 17509675. S2CID 3238035.
- ^ Luo Y, Nie J, Gong QH, Lu YF, Wu Q, Shi JS (2007). "Protective effects of icariin against learning and memory deficits induced by aluminium in rats". Clin. Exp. Pharmacol. Physiol. 34 (8): 792–795. doi:10.1111/j.1440-1681.2007.04647.x. PMID 17600559. S2CID 20134331.
- ^ Zheng M, Qu L, Lou Y (2008). "Effects of icariin combined with Panax notoginseng saponins on ischemia reperfusion-induced cognitive impairments related with oxidative stress and CA1 of hippocampal neurons in rat". Phytother. Res. 22 (5): 597–604. doi:10.1002/ptr.2276. PMID 18398927. S2CID 11444795.
- ^ XiaoangLi et. al, "Icariin enhances youth-like features by attenuating the declined gut microbiota in the aged mice", Pharmacological Research, vol. 168, June 2021
- PDE5 inhibitors
- Flavonol glucosides
- Flavonol rhamnosides
- Prenylflavonoids
- Phenol ethers