Propentofylline
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.133.568 |
| Chemical and physical data | |
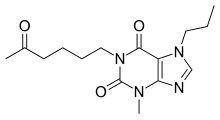
| Formula | C15H22N4O3 |
| Molar mass | 306.366 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Propentofylline (HWA 285) is a xanthine derivative drug with purported neuroprotective effects.
Pharmacology[]
It is a phosphodiesterase inhibitor,[1] and also acts as an adenosine reuptake inhibitor.[1][2][3]
Uses[]
Propentofylline was studied as a possible treatment for Alzheimer's disease and multi-infarct dementia,[4][5] and has been studied, to a lesser extent, as a possible adjunct in the treatment of ischemic stroke, due to its vasodilating properties.[6][7]
Propentofylline is in use as a veterinary medicine in older dogs.[8]
See also[]
References[]
- ^ a b Frampton M, Harvey RJ, Kirchner V (2003). Frampton MA (ed.). "Propentofylline for dementia". Cochrane Database Syst Rev (2): CD002853. doi:10.1002/14651858.CD002853. PMID 12804440.
- ^ Salimi S, Fotouhi A, Ghoreishi A, et al. (April 2008). "A placebo controlled study of the propentofylline added to risperidone in chronic schizophrenia". Prog. Neuropsychopharmacol. Biol. Psychiatry. 32 (3): 726–732. doi:10.1016/j.pnpbp.2007.11.021. PMID 18096287.
- ^ Numagami Y, Marro PJ, Mishra OP, Delivoria-Papadopoulos M (June 1998). "Effect of propentofylline on free radical generation during cerebral hypoxia in the newborn piglet". Neuroscience. 84 (4): 1127–1133. doi:10.1016/S0306-4522(97)00542-3. PMID 9578400.
- ^ Frampton M, Harvey RJ, Kirchner V (2003). Frampton MA (ed.). "Propentofylline for dementia". Cochrane Database of Systematic Reviews (2): CD002853. doi:10.1002/14651858.CD002853. PMID 12804440.
- ^ Kittner B, Rössner M, Rother M (1997). "Clinical trials in dementia with propentofylline". Ann. N. Y. Acad. Sci. 826: 307–316. doi:10.1111/j.1749-6632.1997.tb48481.x. PMID 9329701.
- ^ Bath PM, Bath-Hextall FJ (2004). Bath PM (ed.). "Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke". Cochrane Database of Systematic Reviews (3): CD000162. doi:10.1002/14651858.CD000162.pub2. PMID 15266424.
- ^ Huber M, Kittner B, Hojer C, Fink GR, Neveling M, Heiss WD (1993). "Effect of propentofylline on regional cerebral glucose metabolism in acute ischemic stroke". J. Cereb. Blood Flow Metab. 13 (3): 526–30. doi:10.1038/jcbfm.1993.68. PMID 8478410.
- ^ "Launch of tablet to make life easier for dogs in old age". Yorkshire Post. 7 September 2012. Retrieved 28 December 2019.
Categories:
- Xanthines
- Ketones
- PDE4 inhibitors
- Adenosine reuptake inhibitors
- Adenosine receptor antagonists
- Nervous system drug stubs