1-Phenylethylamine

| |
| Names | |
|---|---|
| Preferred IUPAC name
1-Phenylethan-1-amine | |
Other names
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.588 |
| KEGG | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| Properties | |
Chemical formula
|
C8H11N |
| Molar mass | 121.183 g·mol−1 |
| Density | 0.94 g/mL |
| Melting point | -65 C[citation needed] |
| Boiling point | 187 °C (369 °F; 460 K) |
| Hazards | |
| Main hazards | Corrosive |
| Related compounds | |
Related stereoisomers
|
(R)-(+)- (CAS [3886-69-9]) (S)-(–)- (CAS [2627-86-3]) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1-Phenylethylamine is the organic compound with the formula C6H5CH(NH2)CH3. Classified as a monoamine, this colorless liquid is often used in chiral resolutions. Like benzylamine, it is highly basic and forms stable ammonium salts and imines.
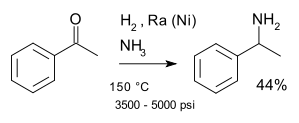
This compound may be prepared by the reductive amination of acetophenone. One major route for this chemical uses the Mignonac reaction, a one-pot protocol using hydrogen gas as the reducing agent:[1]
The Leuckart reaction, using ammonium formate, is another method for this transformation.[2]
L-malic acid is used to resolve 1-Phenylethylamine, a versatile resolving agent in its own right. The dextrorotatory enantiomer crystallizes with the malate, leaving the levorotatory form in solution.[3]
See also[]
- 2-Phenylethylamine
References[]
- ^ John C. Robinson, Jr. and H. R. Snyder (1943). "α-Phenylethylamine". Organic Syntheses. 23: 68. doi:10.15227/orgsyn.023.0068.
- ^ Mann, F. G.; Saunders, B. C. (1960). Practical Organic Chemistry, 4th Ed. London: Longman. pp. 223–224. ISBN 9780582444072.
- ^ A. W. Ingersoll (1937). "d- and l-α-Phenylethylamine". Organic Syntheses. 17: 80. doi:10.15227/orgsyn.017.0080.
Categories:
- Phenethylamines