Amastatin ATC code
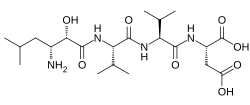
(2S )-2-[[(2S )-2-[[(2S )-2-[[(2S ,3R )-3-Amino-2-hydroxy-5-methylhexanoyl]amino]-3-methylbutanoyl]amino]-3-methylbutanoyl]amino]butanedioic acid
CAS Number PubChem CID ChemSpider KEGG CompTox Dashboard (EPA ) ECHA InfoCard 100.131.532 Formula C 21 H 38 N 4 O 8 Molar mass −1 3D model (JSmol )
CC(C)CC(C(C(=O)NC(C(C)C)C(=O)NC(C(C)C)C(=O)NC(CC(=O)O)C(=O)O)O)N
InChI=1S/C21H38N4O8/c1-9(2)7-12(22)17(28)20(31)25-16(11(5)6)19(30)24-15(10(3)4)18(29)23-13(21(32)33)8-14(26)27/h9-13,15-17,28H,7-8,22H2,1-6H3,(H,23,29)(H,24,30)(H,25,31)(H,26,27)(H,32,33)/t12-,13+,15+,16+,17+/m1/s1
Key:QFAADIRHLBXJJS-ZAZJUGBXSA-N
Amastatin , also known as 3-amino-2-hydroxy-5-methylhexanoyl-L -valyl-L -valyl-L -aspartic acid , is a naturally occurring , competitive and reversible aminopeptidase inhibitor that was isolated from Streptomyces sp. ME 98-M3[1] leucyl aminopeptidase , alanyl aminopeptidase (aminopeptidase M/N), bacterial leucyl aminopeptidase (Aeromonas proteolytica aminopeptidase), leucyl/cystinyl aminopeptidase (oxytocinase/vasopressinase),[2] glutamyl aminopeptidase (aminopeptidase A),[3] [4] arginyl aminopeptidase (aminopeptidase B).[5] [6] central nervous system effects of oxytocin and vasopressin in vivo .[7] met-enkephalin , dynorphin A , and other endogenous peptides .[8]
See also [ ] References [ ]
^ John Buckingham (2 December 1993). Dictionary of Natural Products ISBN 978-0-412-46620-5 ^ Nakanishi Y, Nomura S, Okada M, Ito T, Katsumata Y, Kikkawa F, Hattori A, Tsujimoto M, Mizutani S (2000). "Immunoaffinity purification and characterization of native placental leucine aminopeptidase/oxytocinase from human placenta". Placenta . 21 (7): 628–34. doi :10.1053/plac.2000.0564 . PMID 10985965 . ^ Peter Boger; Gerhard Sandmann (31 July 1989). Target Sites of Herbicide Action ISBN 978-0-8493-4985-0 ^ Thomas Scott; Eric Ian Mercer (1997). Concise Encyclopedia Biochemistry and Molecular Biology 35 –. ISBN 978-3-11-014535-9 ^ Hamao Umezawa (9 May 2014). Small Molecular Immunomodifiers of Microbial Origin: Fundamental and Clinical Studies of Bestatin ISBN 978-1-4831-9033-4 ^ Graham Barrett (6 December 2012). Chemistry and Biochemistry of the Amino Acids ISBN 978-94-009-4832-7 ^ Meisenberg G, Simmons WH (1984). "Amastatin potentiates the behavioral effects of vasopressin and oxytocin in mice". Peptides . 5 (3): 535–9. doi :10.1016/0196-9781(84)90083-4 . PMID 6540873 . S2CID 3881661 . ^ Oka T, Hiranuma T, Liu XF, Ohgiya N, Iwao K, Matsumiya T (1993). "[Enkephalin-inactivating enzymes]" . Nippon Yakurigaku Zasshi (in Japanese). 101 (4): 197–207. doi :10.1254/fpj.101.4_197 PMID 8390390 .
Adiponectin
AdipoR1 AdipoR2
Agonists: Peptide: Adiponectin ; Non-peptide: AdipoRon
Deoxyschizandrin Parthenolide Taxifoliol
Angiotensin Bradykinin
Agonists: Bradykinin Kallidin Antagonists: Icatibant LF22-0542 CGRP
Agonists: Amylin CGRP Pramlintide Antibodies: Eptinezumab Erenumab Fremanezumab Galcanezumab Cholecystokinin
CCKA
Agonists: Cholecystokinin CCKB
Agonists: Cholecystokinin CCK-4 Gastrin Pentagastrin (CCK-5) Unsorted
CRH
CRF1
Agonists: Corticorelin Corticotropin-releasing hormone Sauvagine Urocortin CRF2
Agonists: Corticorelin Corticotropin-releasing hormone Sauvagine Urocortin
Cytokine See here instead.
Endothelin
Agonists: Endothelin 1 Endothelin 2 Endothelin 3 Sarafotoxin Galanin
GAL1 GAL2 GAL3
Agonists: Galanin Galmic Galnon
Ghrelin/GHS GH GHRH GLP
GLP-1 GLP-2
Agonists: GLP-2 Teduglutide Others
Glucagon GnRH Gonadotropin Growth factor See here instead.
Insulin
Agonists: Chaetochromin (4548-G05) Insulin-like growth factor 1 Insulin-like growth factor 2 Insulin Insulin aspart Insulin degludec Insulin detemir Insulin glargine Insulin glulisine Insulin lispro Mecasermin Mecasermin rinfabate Kinase inhibitors: Linsitinib Antibodies: (against IGF-1 and IGF-2) Kisspeptin Leptin
Agonists: Leptin Metreleptin MCH
MCH1
Agonists: Melanin-concentrating hormone MCH2
Agonists: Melanin-concentrating hormone
Melanocortin Neuropeptide FF
Agonists: Neuropeptide FF Neuropeptide S Neuropeptide Y
Y1
Agonists: Neuropeptide Y Peptide YY Y2
Agonists: Neuropeptide Y Peptide YY Y4
Agonists: Neuropeptide Y Pancreatic polypeptide Peptide YY Y5
Agonists: Neuropeptide Y Peptide YY
Neurotensin
NTS1
Agonists: Neurotensin Neuromedin N NTS2
Opioid See here instead.
Orexin Oxytocin Prolactin PTH
Agonists: Abaloparatide Parathyroid hormone Parathyroid hormone-related protein (PTHrP) Teriparatide Relaxin
Agonists: Insulin-like factor 3 Relaxin (, , 3 )Serelaxin Somatostatin Tachykinin TRH TSH
Agonists: Thyrotropin alfa TSH (thyrotropin) Vasopressin VIP /PACAP
VIPR1
Agonists: Peptide: PACAP VIP VIPR2 PAC1 Unsorted
Others
Endogenous: Adrenomedullin Apelin Asprosin Bombesin Calcitonin Carnosine CART CLIP DSIP Enteroglucagon Formyl peptide GALP GIP GRP Integrin ligands (collagens , fibrinogen , fibronectin , laminins , ICAM-1 , ICAM-2 , osteopontin , VCAM-1 , vitronectin )Kininogens Motilin Natriuretic peptides (ANP , BNP , CNP , urodilatin )Nesfatin-1 Neuromedin B Neuromedin N Neuromedin S Neuromedin U Obestatin Osteocalcin Resistin Secretin Thymopoietin Thymosins Thymulin Urotensin-II VGF Exogenous: Lifitegrast (LFA-1 antagonist)
See also
Receptor/signaling modulators
MOR
Unknown/unsorted: Cannabidiol Coronaridine Cyproterone acetate Tabernanthine Tetrahydrocannabinol DOR
Unknown/unsorted: 18-MC Cannabidiol Coronaridine Cyproterone acetate Tabernanthine Tetrahydrocannabinol KOR
Unknown/unsorted: Akuammicine Akuammine Coronaridine Cyproterone acetate Ibogamine Tabernanthine NOP Unsorted
β-Casomorphins Amidorphin Cytochrophin-4 Gliadorphin (gluteomorphin) Gluten exorphins Hemorphins Kava constituentsNEM Neoendorphins Nepetalactone (catnip )Rubiscolins Others
Propeptides: β-Lipotropin (proendorphin) Prodynorphin Proenkephalin Proopiomelanocortin (POMC) Others: Kyotorphin (met-enkephalin releaser/degradation stabilizer)