From Wikipedia, the free encyclopedia
opioid analgesic
Eptazocine Routes of Oral ATC code Legal status
In general: ℞ (Prescription only)
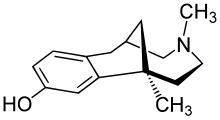
(1S ,6S )-1,4-dimethyl-2,3,4,5,6,7-hexahydro-1H -1,6-methano-4-benzazonin-10-ol
CAS Number PubChem CID ChemSpider UNII ChEMBL Formula C 15 H 21 N O Molar mass −1 3D model (JSmol )
Oc1ccc2c(c1)[C@@]3(CCN(C[C@H](C2)C3)C)C
InChI=1S/C15H21NO/c1-15-5-6-16(2)10-11(9-15)7-12-3-4-13(17)8-14(12)15/h3-4,8,11,17H,5-7,9-10H2,1-2H3/t11-,15-/m1/s1
Key:ZOWQTJXNFTWSCS-IAQYHMDHSA-N
Eptazocine (Sedapain ) is an opioid analgesic which was introduced in Japan by Morishita in 1987.[1] [2] [3] [4] κ-opioid receptor agonist and μ-opioid receptor antagonist .[4] [5] [6]
See also [ ] References [ ]
^ Index nominum 2000: international drug directory ISBN 978-3-88763-075-1 . Retrieved 29 November 2011 .^ American Chemical Society. Division of Medicinal Chemistry (1990). Annual Reports in Medicinal Chemistry ISBN 978-0-12-040525-1 . Retrieved 29 November 2011 . ^ Nabeshima T, Matsuno K, Kamei H, Kameyama T (May 1985). "The interaction of eptazocine, a novel analgesic, with opioid receptors". Research Communications in Chemical Pathology and Pharmacology . 48 (2): 173–81. PMID 2992058 . ^ a b Hiroshi Nagase; Silvia N. Calderon (21 January 2011). Chemistry of Opioids ISBN 978-3-642-18106-1 . Retrieved 29 November 2011 . ^ Tamura T, Ogawa J, Taniguchi T, Waki I (January 1990). "[Preferential action of eptazocine, a novel analgesic, with opioid receptors in isolated guinea pig ileum and mouse vas deferens preparations]" . Nihon Yakurigaku Zasshi. Folia Pharmacologica Japonica (in Japanese). 95 (1): 41–6. doi :10.1254/fpj.95.1_41 PMID 2154395 . ^ Ian Morton; Ian K. M. Morton; Judith M. Hall (1999). Concise dictionary of pharmacological agents: properties and synonyms ISBN 978-0-7514-0499-9 . Retrieved 29 November 2011 .
Analgesics (N02A , N02B )
Opioids
Opiates /opium
Codeine #
Morphine # (+naltrexone )Opium Laudanum Paregoric Semisynthetic Synthetic
Paracetamol -typeNSAIDs
Propionates Oxicams
Isoxicam Lornoxicam Meloxicam Piroxicam Tenoxicam Acetates COX-2 inhibitors Fenamates Salicylates Pyrazolones
Aminophenazone ‡ Ampyrone Metamizole (dipyrone) Nifenazone Phenazone Propyphenazone (+paracetamol/caffeine ) Others
Cannabinoids
Cannabidiol Cannabis Nabilone Nabiximols Tetrahydrocannabinol (dronabinol) Ion channel
Calcium blockers
Alcohol (ethanol) Gabapentin Gabapentin enacarbil Leconotide Mirogabalin Pregabalin Ziconotide Sodium blockers
Carbamazepine Lacosamide Local anesthetics (e.g., cocaine , lidocaine )Mexiletine Nefopam Tricyclic antidepressants (e.g., amitriptyline # )Nav 1.7/1.8-selective: DSP-2230 § Funapide § PF-05089771 § Potassium openers
Myorelaxants
Carisoprodol Chlorzoxazone Cyclobenzaprine Mephenoxalone Methocarbamol Orphenadrine Others
# WHO-EM ‡ Withdrawn from marketClinical trials :
† Phase III § Never to phase III
MOR
Unknown/unsorted: Cannabidiol Coronaridine Cyproterone acetate Tabernanthine Tetrahydrocannabinol DOR
Unknown/unsorted: 18-MC Cannabidiol Coronaridine Cyproterone acetate Tabernanthine Tetrahydrocannabinol KOR
Unknown/unsorted: Akuammicine Akuammine Coronaridine Cyproterone acetate Ibogamine Tabernanthine NOP Unsorted
β-Casomorphins Amidorphin Cytochrophin-4 Gliadorphin (gluteomorphin) Gluten exorphins Hemorphins Kava constituentsNEM Neoendorphins Nepetalactone (catnip )Rubiscolins Others
Others: Kyotorphin (met-enkephalin releaser/degradation stabilizer)
Categories :
Drugs not assigned an ATC code Phenols Analgesics Opioids Hidden categories:
CS1 Japanese-language sources (ja) Articles with short description Pages with lower-case short description Short description is different from Wikidata Drugs with non-standard legal status Chemical pages without DrugBank identifier Articles without KEGG source Articles containing unverified chemical infoboxes