Fluorenol[1]
Names
IUPAC name
9H -Fluoren-9-ol
Other names
9-Hydroxyfluorene
Identifiers
CAS Number
3D model (JSmol )
ChEBI
ChemSpider
ECHA InfoCard 100.015.345
EC Number
UNII
InChI=1S/C13H10O/c14-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)13/h1-8,13-14H
N Key: AFMVESZOYKHDBJ-UHFFFAOYSA-N
N InChI=1/C13H10O/c14-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)13/h1-8,13-14H
Key: AFMVESZOYKHDBJ-UHFFFAOYAM
C1=CC=C2C(=C1)C(C3=CC=CC=C32)O
Properties
Chemical formula
C13 H10 O
Molar mass
182.22 g/mol
Appearance
Off-white crystalline powder
Density
1.151 g/mL
Melting point
152 to 155 °C (306 to 311 °F; 425 to 428 K)
Solubility in water
Practically insoluble [2]
Hazards
NFPA 704
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
N what is Y N
Infobox references
Chemical compound
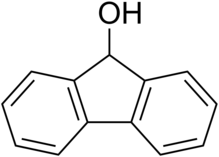
Fluorenol is an alcohol derivative of fluorene . In the most significant isomer, fluoren-9-ol or 9-hydroxyfluorene, the hydroxy group is located on the bridging carbon between the two benzene rings. Hydroxyfluorene can be converted to fluorenone by oxidation. It is a white-cream colored solid at room temperature.
Toxicity [ ] Fluorenol is toxic to aquatic organisms including algae, bacteria, and crustaceans.[3] insecticide in 1939,[4] algaecide against the green algae Dunaliella [5]
Its toxicity and carcinogenicity in humans are unknown.[5]
Eugeroic [ ] A study published by Cephalon describing research to develop a successor to the eugeroic modafinil reported that the corresponding fluorenol derivative was 39% more effective than modafinil at keeping mice awake over a 4-hour period.[6] active metabolite , which they identify as fluorenol itself.[6] dopamine reuptake inhibitor with an IC50 of 9 μM , notably 59% weaker than modafinil (IC50 = 3.70 μM),[6] addiction .[7] cytochrome P450 2C19 , unlike modafinil.[6]
See also [ ] Adrafinil Armodafinil CRL-40,940 CRL-40,941 Fluorenone References [ ]
^ 9-Hydroxyfluorene , chemicalland21.com^ Record of 9H-Fluoren-9-ol in the GESTIS Substance Database of the Institute for Occupational Safety and Health , accessed on 5 November 2008.
^ Šepič, Ester; Bricelj, Mihael; Leskovšek, Hermina (2003). "Toxicity of fluoranthene and its biodegradation metabolites to aquatic organisms". Chemosphere . 52 (7): 1125–33. doi :10.1016/S0045-6535(03)00321-7 . PMID 12820993 . ^ US patent 2197249: Insecticide ^ a b MSDS Archived 2016-03-04 at the Wayback Machine ^ a b c d Dunn, D.; Hostetler, G.; Iqbal, M.; Marcy, V. R.; Lin, Y. G.; Jones, B.; Aimone, L. D.; Gruner, J.; Ator, M. A.; Bacon, E. R.; Chatterjee, S. (2012). "Wake promoting agents: Search for next generation modafinil, lessons learned: Part III". Bioorganic & Medicinal Chemistry Letters . 22 (11): 3751–3753. doi :10.1016/j.bmcl.2012.04.031 . PMID 22546675 . ^ Wise, R. A. (1996). "Neurobiology of addiction". Current Opinion in Neurobiology . 6 (2): 243–51. doi :10.1016/S0959-4388(96)80079-1 . PMID 8725967 .
Stimulants
Adamantanes
Adapromine Amantadine Bromantane Memantine Rimantadine Adenosine antagonists Alkylamines
Cyclopentamine Cypenamine Cyprodenate Heptaminol Isometheptene Levopropylhexedrine Methylhexaneamine Octodrine Propylhexedrine Tuaminoheptane Ampakines Arylcyclohexylamines Benzazepines
6-Br-APB SKF-77434 SKF-81297 SKF-82958 Cathinones Cholinergics Convulsants Eugeroics Oxazolines Phenethylamines Phenylmorpholines Piperazines
2C-B-BZP 3C-PEP BZP CM156 DBL-583 GBR-12783 GBR-12935 GBR-13069 GBR-13098 GBR-13119 MeOPP MBZP oMPP Vanoxerine Piperidines Pyrrolidines Racetams
Oxiracetam Phenylpiracetam Phenylpiracetam hydrazide Tropanes Tryptamines Others ATC code : N06B
Pest control : herbicides
Anilides /anilines
acetochlor alachlor asulam benfluralin butachlor dimethenamid metolachlor oryzalin pendimethalin propachlor propanil trifluralin Aromatic acids
aminopyralid chloramben clopyralid dicamba picloram quinclorac Arsenicals
cacodylic acid copper arsenate DSMA MSMA HPPD inhbitors
mesotrione nitisinone leptospermone sethoxydim Organophosphorus
bensulide bialaphos ethephon glufosinate glyphosate Phenoxy
Auxins
2,4-D 2,4-DB dichlorprop fenoprop MCPA MCPB mecoprop 2,4,5-T ACCase inhibitors
FOP herbicides
diclofop chlorazifop fenoxaprop fluazifop haloxyfop quizalofop DIM herbicides
Protox inhibitors
Nitrophenyl ethers
acifluorfen bifenox fluorodifen fomesafen lactofen nitrofen oxyfluorfen Pyrimidinediones Triazolinones
Pyridines
dithiopyr fluroxypyr imazapyr triclopyr Quaternary
Triazines
Ureas
Others
3-AT aclonifen aminocyclopyrachlor Bentazon bromoxynil clomazone DCBN dinoseb indaziflam juglone methazole metam sodium metribuzin pyribenzoxim Ziram
Monoamine reuptake inhibitors
DAT (DRIs
Piperazines: DBL-583 GBR-12783 GBR-12935 GBR-13069 GBR-13098 Nefazodone Vanoxerine
Piperidines: 4-Fluoropethidine Benocyclidine (BTCP) Desoxypipradrol Dexmethylphenidate Difemetorex Ethylphenidate HDMP-28 Methylphenidate Pethidine (meperidine) Phencyclidine Pipradrol Serdexmethylphenidate Tenocyclidine
Pyrrolidines: Diphenylprolinol MDPV Naphyrone Prolintane Pyrovalerone
Tropanes: Altropane Benzatropine (benztropine) Brasofensine CFT Cocaine Dichloropane Difluoropine Etybenzatropine (ethybenztropine) FE-β-CPPIT FP-β-CPPIT Ioflupane (123 I) RTI-55 RTI-112 RTI-113 RTI-121 RTI-126 RTI-150 RTI-177 RTI-229 RTI-336 Tesofensine Troparil Tropoxane WF-11 WF-23 WF-31 WF-33
NET (NRIs
Norepinephrine–dopamine reuptake inhibitors: Amineptine Bupropion Fencamine Fencamfamin Hydroxybupropion Lefetamine Levophacetoperane LR-5182 Manifaxine Methylphenidate Nomifensine O-2172 Radafaxine Serdexmethylphenidate Solriamfetol
Tricyclic antidepressants: Amitriptyline Butriptyline Cianopramine Clomipramine Desipramine Dosulepin (dothiepin) Doxepin Imipramine Lofepramine Melitracen Nortriptyline Protriptyline Trimipramine
Tetracyclic antidepressants: Amoxapine Maprotiline Mianserin Oxaprotiline Setiptiline
Others: Antihistamines (e.g., brompheniramine , chlorphenamine , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., ketamine , phencyclidine )Dopexamine Ephenidine Ginkgo biloba Indeloxazine Nefazodone Opioids (e.g., desmetramadol , methadone , pethidine (meperidine) , tapentadol , tramadol , levorphanol )
SERT (SRIs
Selective serotonin reuptake inhibitors and serotonin receptor modulators: Etoperidone Litoxetine Lubazodone LY-393558 Quipazine SB-649915 TGBA01AD Trazodone Vilazodone Vortioxetine
Serotonin–norepinephrine reuptake inhibitors: Atomoxetine (tomoxetine) Bicifadine Desvenlafaxine Duloxetine Eclanamine Levomilnacipran McN5652 Milnacipran N-Methyl-PPPA PPPA Tofenacin Venlafaxine
Tricyclic antidepressants: Amitriptyline Cianopramine Clomipramine Cyanodothiepin Desipramine Dosulepin (dothiepin) Doxepin Imipramine Lofepramine Nortriptyline Pipofezine Protriptyline
Others: Amoxapine Antihistamines (e.g., brompheniramine , chlorphenamine , dimenhydrinate , diphenhydramine , mepyramine (pyrilamine) , pheniramine , tripelennamine )Antipsychotics (e.g., loxapine , ziprasidone )Arylcyclohexylamines (e.g., 3-MeO-PCP , esketamine , ketamine , methoxetamine , phencyclidine )Cyclobenzaprine Delucemine Dextromethorphan Dextrorphan Efavirenz Hypidone Medifoxamine Mesembrine Mifepristone MIN-117 (WF-516) N-Me-5-HT Opioids (e.g., dextropropoxyphene , methadone , pethidine (meperidine) , levorphanol , tapentadol , tramadol )Roxindole
VMATs
Amiodarone Amphetamines (e.g., amphetamine , methamphetamine , MDMA )Bietaserpine Deserpidine Deutetrabenazine Dihydrotetrabenazine Efavirenz GBR-12935 Ibogaine Ketanserin Lobeline Reserpine Rose bengal Tetrabenazine Valbenazine Vanoxerine (GBR-12909) Others
DAT modulators: Agonist-like: SoRI-9804 ; Antagonist-like: SoRI-20041 See also: Receptor/signaling modulators • Monoamine releasing agents • Adrenergics • Dopaminergics • Serotonergics • Monoamine metabolism modulators • Monoamine neurotoxins