Chemical compound
Oleamide
Names
Preferred IUPAC name
Other names
Oleylamide
Identifiers
CAS Number
3D model (JSmol )
ChEBI
ChEMBL
ChemSpider
ECHA InfoCard 100.005.550
EC Number
IUPHAR/BPS
UNII
InChI=1S/C18H35NO/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h9-10H,2-8,11-17H2,1H3,(H2,19,20)/b10-9-
Y Key: FATBGEAMYMYZAF-KTKRTIGZSA-N
Y InChI=1/C18H35NO/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h9-10H,2-8,11-17H2,1H3,(H2,19,20)/b10-9-
Key: FATBGEAMYMYZAF-KTKRTIGZBR
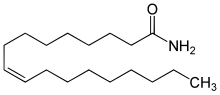
O=C(N)CCCCCCC\C=C/CCCCCCCC
Properties
Chemical formula
C 18 H 35 N O
Molar mass
−1
Appearance
Creamy solid[1]
Density
0.879 g/cm3
Melting point
70 °C (158 °F; 343 K)[2] [3]
Boiling point
> 200 °C (392 °F; 473 K)[1]
Solubility in water
Insoluble[1]
Hazards
NFPA 704
Flash point
> 200 °C (392 °F; 473 K)[1]
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
N what is Y N
Infobox references
Chemical compound
Oleamide is an organic compound with the formula CH3 (CH2 )7 CH=CH(CH2 )7 CONH2 (.[4] amide derived from the fatty acid oleic acid . It is a colorless waxy solid and occurs in nature. Sometimes labeled as a fatty acid primary amide (FAPA), it is biosynthesized from N-oleoylglycine.[5]
Biochemical and medical aspects [ ] In terms of natural occurrence, oleamide was first detected in human plasma. It was later shown to accumulate in the cerebrospinal fluid during sleep deprivation and induces sleep in animals .[5] [6]
It has been considered as a potential treatment for mood and sleep disorders, as well as cannabinoid -regulated depression.[7] [8]
In terms of its sleep inducing effects, it is speculated that oleamide interacts with multiple neurotransmitter systems.[9]
Other occurrences [ ] Synthetic oleamide has a variety of industrial uses including as a slip agent, a lubricant , and a corrosion inhibitor .[10]
Oleamide was found to be leaking out of polypropylene plastics in laboratory experiments, affecting experimental results.[11] yogurt , the problem is being studied.[12]
Oleamide is "one of the most frequent non-cannabinoid ingredients associated with Spice products."[13] [14]
See also [ ] Anandamide Fatty acid amide hydrolase Virodhamine References [ ]
^ a b c d Oleamide at chemicalland21.com^ "Oleamide CAS#: 301-02-0" .^ "(9Z)-9-Octadecenamide | C18H35NO | ChemSpider" .^ "Oleamide" .^ a b McKinney, Michele K.; Cravatt, Benjamin F. (June 2005). "Structure and Function of Fatty Acid Amide Hydrolase" . Annual Review of Biochemistry . 74 (1): 411–432. doi :10.1146/annurev.biochem.74.082803.133450 . PMID 15952893 . S2CID 23571858 . ^ Cravatt, B.; Prospero-Garcia, O; Siuzdak, G; Gilula, N.; Henriksen, S.; Boger, D.; Lerner, R. (9 June 1995). "Chemical characterization of a family of brain lipids that induce sleep" . Science . 268 (5216): 1506–1509. Bibcode :1995Sci...268.1506C . doi :10.1126/science.7770779 . PMID 7770779 . S2CID 32070839 . ^ Methods of treating anxiety and mood disorders with oleamide – US Patent 6359010 Archived 2011-06-12 at the Wayback Machine ^ Mechoulam, Raphael; Fride, Ester; Hanu, Lumir; Sheskin, Tzviel; Bisogno, Tiziana; Di Marzo, Vincenzo; Bayewitch, Michael; Vogel, Zvi (September 1997). "Anandamide may mediate sleep induction". Nature . 389 (6646): 25–26. Bibcode :1997Natur.389R..25M . doi :10.1038/37891 . PMID 9288961 . S2CID 26371103 . ^ Fedorova, I; Hashimoto, A; Fecik, RA; Hedrick, MP; Hanus, LO; Boger, DL; Rice, KC; Basile, AS (October 2001). "Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems". The Journal of Pharmacology and Experimental Therapeutics . 299 (1): 332–42. PMID 11561096 . ^ Surfactants : Westco Oleamide a Slip Agent In Polyethylene Films Archived January 27, 2007, at the Wayback Machine ^ McDonald, G. R.; Hudson, A. L.; Dunn, S. M. J.; You, H.; Baker, G. B.; Whittal, R. M.; Martin, J. W.; Jha, A.; Edmondson, D. E.; Holt, A. (7 November 2008). "Bioactive Contaminants Leach from Disposable Laboratory Plasticware". Science . 322 (5903): 917. Bibcode :2008Sci...322..917M . doi :10.1126/science.1162395 . PMID 18988846 . S2CID 35526901 . ^ Mittelstaedt, Martin (6 November 2008). "Researchers Raise Alarm After Chemical Leak Found In Common Plastic" . Globe and Mail . Retrieved 10 June 2013 . ^ Fattore, Liana; Fratta, Walter (21 September 2011). "Beyond THC: The New Generation of Cannabinoid Designer Drugs" . Frontiers in Behavioral Neuroscience . 5 : 60. doi :10.3389/fnbeh.2011.00060 PMC 3187647 PMID 22007163 . ^ Uchiyama, Nahoko; Kikura-Hanajiri, Ruri; Ogata, Jun; Goda, Yukihiro (May 2010). "Chemical analysis of synthetic cannabinoids as designer drugs in herbal products". Forensic Science International . 198 (1–3): 31–38. doi :10.1016/j.forsciint.2010.01.004 . PMID 20117892 .
Cannabinoids
Phytocannabinoids
Cannabichromenes Cannabicyclols Cannabidiols Cannabielsoins Cannabigerols Cannabinols and cannabinodiols Cannabitriols Delta-8-tetrahydrocannabinols Delta-9-tetrahydrocannabinols
Delta-9-THC (THC)
THCH THCP
THCV
Miscellaneous cannabinoids Active metabolites
Endocannabinoids
Arachidonoyl ethanolamide (AEA; anandamide) 2-Arachidonoylglycerol (2-AG) 2-Arachidonyl glyceryl ether (2-AGE; noladin ether) 2-Oleoylglycerol (2-OG) N-Arachidonoyl dopamine (NADA) N-Arachidonylglycine (NAGly) N-Arachidonoyl serotonin (AA-5-HT) Docosatetraenoylethanolamide (DEA) Lysophosphatidylinositol (LPI) Oleamide Oleoylethanolamide (OEA) Palmitoylethanolamide (PEA) RVD-Hpα Stearoylethanolamide (SEA) O-Arachidonoyl ethanolamine (O-AEA; virodhamine) Synthetic
Classical cannabinoids Non-classical Adamantoylindoles Benzimidazoles Benzoylindoles
AM-630 AM-679 AM-694 AM-1241 AM-2233 GW-405,833 (L-768,242) Pravadoline RCS-4 WIN 54,461 Cyclohexylphenols Eicosanoids Hydrocarbons Indazole carboxamides Indazole-3- Indole-3-carboxamides Indole-3-carboxylates Naphthoylindazoles Naphthoylindoles Naphthoylpyrroles Naphthylmethylindenes
AM-2201 AM-694 WIN-55,212-2 Naphthylmethylindoles Phenylacetylindoles Pyrazolecarboxamides Pyrrolobenzoxazines Quinolinyl esters Tetramethylcyclo- Tetramethylcyclo-
A-796,260 A-834,735 FUB-144 UR-144 XLR-11 XLR-12 Tetramethylcyclo- Others
Allosteric CBR ligands Endocannabinoid (inactivation inhibitors) Anticannabinoids (antagonists/inverse
See also: Cannabinoid receptor modulators (cannabinoids by pharmacology)List of: AM cannabinoids JWH cannabinoids Designer drugs § Synthetic cannabimimetics
Neurotransmitters
Amino acid -derived
Major excitatory /
Glutamate system
Agmatine Aspartic acid (aspartate) Glutamic acid (glutamate) Glutathione Glycine GSNO GSSG Kynurenic acid NAA NAAG Proline Serine GABA system Glycine system
α-Alanine β-Alanine Glycine Hypotaurine Proline Sarcosine Serine Taurine GHB system
Biogenic amines
Monoamines
6-OHM Dopamine Epinephrine (adrenaline) NAS (normelatonin) Norepinephrine (noradrenaline) Serotonin (5-HT) Trace amines
3-Iodothyronamine N-Methylphenethylamine N-Methyltryptamine m -Octopaminep -OctopaminePhenylethanolamine Phenethylamine Synephrine Tryptamine m -Tyraminep -Tyramine Others
Neuropeptides
Lipid -derived
Endocannabinoids
2-AG 2-AGE (noladin ether) 2-OG AA-5-HT Anandamide (AEA) DEA LPI NADA NAGly OEA Oleamide PEA RVD-Hpα SEA Virodhamine (O-AEA) Neurosteroids
Nucleobase -derived
Vitamin -derived
Miscellaneous
Gasotransmitters
Carbon monoxide (CO) Hydrogen sulfide (H2 S) Nitric oxide (NO)
Candidates
Acetaldehyde Ammonia (NH3 ) Carbonyl sulfide (COS) Nitrous oxide (N2 O) Sulfur dioxide (SO2 )
Receptor (ligands )
CB1
Agonists (abridged; see here for more) : 2-AG 2-AGE (noladin ether) 11-Hydroxy-THC α-Amyrin · β-Amyrin AB-CHMINACA AM-1220 AM-1221 AM-1235 AM-2201 AM-2232 Anandamide AZ-11713908 Cannabinol CB-13 CP 47,497 CP 55,940 Dimethylheptylpyran DEA ECG EGCG Epicatechin Gallocatechol (gallocatechin) Honokiol HU-210 JWH-007 JWH-015 JWH-018 JWH-073 Kavain L-759,633 Levonantradol Menabitan Nabilone Nabitan NADA O-1812 Oleamide Pravadoline Serinolamide A THC (dronabinol) UR-144 WIN 55,212-2 Yangonin CB2
Agonists: 2-AG 2-AGE (noladin ether) 3,3'-Diindolylmethane 4-O-Methylhonokiol α-Amyrin · β-Amyrin A-796,260 A-834,735 A-836,339 AM-1221 AM-1235 AM-1241 AM-2232 Anandamide AZ-11713908 Cannabinol Caryophyllene CB-13 CBS-0550 CP 55,940 GW-405,833 (L-768,242) GW-842,166X HU-308 JTE 7-31 JWH-007 JWH-015 JWH-018 JWH-133 L-759,633 L-759,656 Magnolol MDA-19 Nabitan NADA Olorinab (APD-371) PF-03550096 S-444,823 SER-601 Serinolamide A UR-144 Tedalinab THC (dronabinol) THCV Virodhamine NAGly GPR18 )
Agonists: Abnormal cannabidiol ACPA AM251 Anandamide Cannabidiol NADGly THC (dronabinol) O-1602 GPR55
Agonists: 2-AGE (noladin ether) Abnormal cannabidiol AM-251 CP 55,940 Lysophosphatidylinositol O-1602 Oleoylethanolamide Palmitoylethanolamide THC (dronabinol) GPR119 Unsorted
Transporter (modulators )
eCBTs
Inhibitors: AM-404 Arachidonoyl serotonin Cannabidiol Guineensine LY-2183240 Paracetamol (acetaminophen) URB-597 VDM-11
Enzyme (modulators )
FAAH MAGL
Inhibitors: IDFP JZL-184 JZL-195 MAFP URB-602 ABHD6 ABHD12
Inhibitors: Betulinic acid Maslinic acid MAFP Oleanolic acid Orlistat (tetrahydrolipstatin) Ursolic acid
Others
Precursors: Phosphatidylethanolamine NAPE Diacylglycerol Others: (directly potentiates activity of 2-AG at CB1 receptor) (FAAH-like anandamide transporter inhibitor)
See also
Receptor/signaling modulators Cannabinoids (cannabinoids by structure)