From Wikipedia, the free encyclopedia
Otenabant ATC code
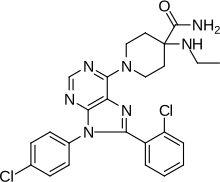
1-[8-(2-chlorophenyl)-9-(4-chlorophenyl)-9H-purin-6-yl]-4-(ethylamino)piperidine-4- carboxamide
CAS Number PubChem CID ChemSpider UNII KEGG ChEMBL CompTox Dashboard (EPA ) Formula C 25 H 25 Cl 2 N 7 O Molar mass −1 3D model (JSmol )
CCNC1(CCN(CC1)C2=NC=NC3=C2N=C(N3C4=CC=C(C=C4)Cl)C5=CC=CC=C5Cl)C(=O)N
InChI=1S/C25H25Cl2N7O/c1-2-31-25(24(28)35)11-13-33(14-12-25)22-20-23(30-15-29-22)34(17-9-7-16(26)8-10-17)21(32-20)18-5-3-4-6-19(18)27/h3-10,15,31H,2,11-14H2,1H3,(H2,28,35)
N Key:UNAZAADNBYXMIV-UHFFFAOYSA-N
N N Y (what is this?)
Otenabant (CP-945,598 ) is a drug which acts as a potent and highly selective CB1 antagonist .[1] Pfizer for the treatment of obesity ,[2] rimonabant .[3]
See also [ ] Cannabinoid receptor antagonist References [ ]
Antiobesity agents /Anorectics (A08 )
Central
Stimulants
4-Methylamphetamine ‡ Amfecloral Amfepentorex Amfepramone Aminorex ‡ Amphetamine Amphetaminil Atomoxetine Benfluorex ‡ Benzphetamine Bupropion (+naltrexone ; +zonisamide† )Cathine Cathinone Chlorphentermine Ciclazindol Clobenzorex Cloforex Clominorex Clortermine Dexfenfluramine ‡ Dextroamphetamine Dexmethylphenidate Difemetorex ‡ Dimethylcathinone Ephedrine Ephedra ‡ Etilamfetamine Etolorex Fenbutrazate Fencamfamin Fenethylline Fenfluramine (+phentermine ‡ )Fenproporex Fludorex Fluminorex Furfenorex ‡ Indanorex Khat Levopropylhexedrine Lisdexamfetamine Manifaxine Mazindol Mefenorex Methamphetamine Methylphenidate Norfenfluramine Pemoline Pentorex Phendimetrazine Phenethylamine Phenmetrazine Phentermine (+topiramate )Phenylpropanolamine Picilorex Pipradrol Prolintane Propylhexedrine Pseudoephedrine Pyrovalerone Radafaxine Reboxetine Setazindol Sibutramine ‡ Synephrine Tesofensine Viloxazine Xylopropamine Zylofuramine Cannabinoid Others
5-HTP Beloranib § Galactomannan
Glucomannan L-DOPA L-Phenylalanine L-Tryptophan L-Tyrosine Lorcaserin Lu AA-33810 Metformin Naltrexone Naloxone Oxyntomodulin P57 Peptide YY Setmelanotide Topiramate Velneperit § Yohimbine (Yohimbe )Zonisamide Water
Peripheral
# WHO-EM ‡ Withdrawn from marketClinical trials :
† Phase III § Never to phase III
Cannabinoids
Phytocannabinoids
Cannabichromenes Cannabicyclols Cannabidiols Cannabielsoins Cannabigerols Cannabinols and cannabinodiols Cannabitriols Delta-8-tetrahydrocannabinols Delta-9-tetrahydrocannabinols
Delta-9-THC (THC)
THCH THCP
THCV
Miscellaneous cannabinoids Active metabolites
Endocannabinoids
Arachidonoyl ethanolamide (AEA; anandamide) 2-Arachidonoylglycerol (2-AG) 2-Arachidonyl glyceryl ether (2-AGE; noladin ether) 2-Oleoylglycerol (2-OG) N-Arachidonoyl dopamine (NADA) N-Arachidonylglycine (NAGly) N-Arachidonoyl serotonin (AA-5-HT) Docosatetraenoylethanolamide (DEA) Lysophosphatidylinositol (LPI) Oleamide Oleoylethanolamide (OEA) Palmitoylethanolamide (PEA) RVD-Hpα Stearoylethanolamide (SEA) O-Arachidonoyl ethanolamine (O-AEA; virodhamine) Synthetic
Classical cannabinoids Non-classical Adamantoylindoles Benzimidazoles Benzoylindoles
AM-630 AM-679 AM-694 AM-1241 AM-2233 GW-405,833 (L-768,242) Pravadoline RCS-4 WIN 54,461 Cyclohexylphenols Eicosanoids Hydrocarbons Indazole carboxamides Indazole-3- Indole-3-carboxamides Indole-3-carboxylates Naphthoylindazoles Naphthoylindoles Naphthoylpyrroles Naphthylmethylindenes Naphthylmethylindoles Phenylacetylindoles Pyrazolecarboxamides Pyrrolobenzoxazines Quinolinyl esters Tetramethylcyclo- Tetramethylcyclo-
A-796,260 A-834,735 FUB-144 UR-144 XLR-11 XLR-12 Tetramethylcyclo- Others
Allosteric CBR ligands Endocannabinoid (inactivation inhibitors) Anticannabinoids (antagonists/inverse
See also: Cannabinoid receptor modulators (cannabinoids by pharmacology)List of: AM cannabinoids JWH cannabinoids Designer drugs § Synthetic cannabimimetics
Receptor (ligands )
CB1
Agonists (abridged; see here for more) : 2-AG 2-AGE (noladin ether) 11-Hydroxy-THC α-Amyrin · β-Amyrin AB-CHMINACA AM-1220 AM-1221 AM-1235 AM-2201 AM-2232 Anandamide AZ-11713908 Cannabinol CB-13 CP 47,497 CP 55,940 Dimethylheptylpyran DEA ECG EGCG Epicatechin Gallocatechol (gallocatechin) Honokiol HU-210 JWH-007 JWH-015 JWH-018 JWH-073 Kavain L-759,633 Levonantradol Menabitan Nabilone Nabitan NADA O-1812 Oleamide Pravadoline Serinolamide A THC (dronabinol) UR-144 WIN 55,212-2 Yangonin CB2
Agonists: 2-AG 2-AGE (noladin ether) 3,3'-Diindolylmethane 4-O-Methylhonokiol α-Amyrin · β-Amyrin A-796,260 A-834,735 A-836,339 AM-1221 AM-1235 AM-1241 AM-2232 Anandamide AZ-11713908 Cannabinol Caryophyllene CB-13 CBS-0550 CP 55,940 GW-405,833 (L-768,242) GW-842,166X HU-308 JTE 7-31 JWH-007 JWH-015 JWH-018 JWH-133 L-759,633 L-759,656 Magnolol MDA-19 Nabitan NADA Olorinab (APD-371) PF-03550096 S-444,823 SER-601 Serinolamide A UR-144 Tedalinab THC (dronabinol) THCV Virodhamine NAGly GPR18 )
Agonists: Abnormal cannabidiol ACPA AM251 Anandamide Cannabidiol NADGly THC (dronabinol) O-1602 GPR55
Agonists: 2-AGE (noladin ether) Abnormal cannabidiol AM-251 CP 55,940 Lysophosphatidylinositol O-1602 Oleoylethanolamide Palmitoylethanolamide THC (dronabinol) GPR119 Unsorted
Transporter (modulators )
eCBTs
Inhibitors: AM-404 Arachidonoyl serotonin Cannabidiol Guineensine LY-2183240 Paracetamol (acetaminophen) URB-597 VDM-11
Enzyme (modulators )
FAAH MAGL
Inhibitors: IDFP JZL-184 JZL-195 MAFP URB-602 ABHD6 ABHD12
Inhibitors: Betulinic acid Maslinic acid MAFP Oleanolic acid Orlistat (tetrahydrolipstatin) Ursolic acid
Others
Precursors: Phosphatidylethanolamine NAPE Diacylglycerol Others: (directly potentiates activity of 2-AG at CB1 receptor) (FAAH-like anandamide transporter inhibitor)
See also
Receptor/signaling modulators Cannabinoids (cannabinoids by structure)
Categories :
Drugs not assigned an ATC code Cannabinoids Purines Piperidines Carboxamides Chloroarenes Pfizer brands CB1 receptor antagonists Cannabinoid stubs Hidden categories:
CS1: long volume value Articles with changed ChemSpider identifier Articles with changed EBI identifier Articles with changed InChI identifier Chemical pages without DrugBank identifier Drugs with no legal status Drugboxes which contain changes to verified fields Drugboxes which contain changes to watched fields All stub articles