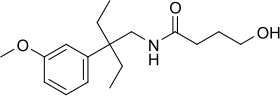
Embutramide
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | intravenous |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.036.149 |
| Chemical and physical data | |
| Formula | C17H27NO3 |
| Molar mass | 293.407 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Embutramide (INN, USAN, BAN) (brand name Embutane) is a potent opioid analgesic and sedative drug that is structurally related to methadone.[1][2][3] It was developed by Hoechst A.G. in 1958[4] and was investigated as a general anesthetic agent, but was found to have a very narrow therapeutic window, with a 50 mg/kg dose producing effective sedation and a 75 mg/kg dose being fatal. Along with strong sedative effects, embutramide also produces respiratory depression and ventricular arrhythmia. Because of these properties, it was never adopted for medical use as an anesthetic as it was considered too dangerous for this purpose. Instead it is used for euthanasia in veterinary medicine, mainly for the euthanization of dogs.
Embutramide is formulated as a combination product under the brand name Tributame, which also contains chloroquine and lidocaine.[5]
Embutramide is used for euthanasia of a range of different animals, mainly small animals kept as pets rather than large farm animals. It may cause significant pain to the animal being euthanized,[6] and so may be less humane than older drugs used for this purpose such as pentobarbital; however, it may have less abuse potential than barbiturates especially in the Tributame combination formulation, and so is less likely to be diverted for recreational abuse.[7] Embutramide has however been reported to be used for suicide by people with access to the drug,[8][9] and was added to the list of Schedule III drugs in the US in 2006, as a Non-Narcotic with ACSCN 2020, which classifies it with depressants such as benzodiazepines, barbiturates, and other sedative-hypnotics.[10]
References[]
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 482–. ISBN 978-1-4757-2085-3.
- ^ Morton IK, Hall JM (31 October 1999). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 108–. ISBN 978-0-7514-0499-9.
- ^ Peterson ME, Talcott PA (7 August 2013). Small Animal Toxicology. Elsevier Health Sciences. pp. 315–. ISBN 978-0-323-24198-4.
- ^ US 3045043, Schmitt K, Henning I, Lindner E, Ott H, "New gamma-hydroxy-carboxylic acid amides and process for their manufacture", issued 17 July 1962, assigned to Hoechst AG
- ^ "TRIBUTAME Euthanasia Solution" (PDF). Freedom of Information Summary NADA 141-245. U.S. Food and Drug Administration. Archived from the original (PDF) on 4 March 2017.
- ^ Hellebrekers LJ, Baumans V, Bertens AP, Hartman W (July 1990). "[The use of T61 for the humane killing of pets and laboratory animals]". Tijdschrift voor Diergeneeskunde (in Dutch and Flemish). 115 (13): 625–32. PMID 2371711.
- ^ "DEA lists embutramide as schedule III controlled substance". Archived from the original on 8 March 2012.
- ^ Smith RA, Lewis D (August 1989). "Suicide by ingestion of T-61". Veterinary and Human Toxicology. 31 (4): 319–20. PMID 2815545.
- ^ Kintz P, Cirimele V, Ludes B (October 2002). "Blood investigation in a fatality involving the veterinary drug T-61". Journal of Analytical Toxicology. 26 (7): 529–31. doi:10.1093/jat/26.7.529. PMID 12423012.
- ^ "DEA lists embutramide as schedule III controlled substance". Journal of the American Veterinary Medical Association. 229 (9): 1358. November 2006. PMID 17139797.
- Drugs not assigned an ATC code
- Anesthetics
- Analgesics
- Hypnotics
- Sedatives
- Phenol ethers
- Carboxamides
- Primary alcohols
- Opioids
- Analgesic stubs