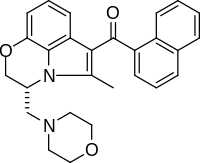
Chemical compound
WIN 55,212-2 Legal status
CA [1] UK :Class B US :Schedule I
show (11R )-2-Methyl-11-[(morpholin-4-yl)methyl]-3-(naphthalene-1-carbonyl)-9-oxa-1-azatricyclo[6.3.1.04,12 ]dodeca-2,4(12),5,7-tetraene
CAS Number PubChem CID IUPHAR/BPS ChemSpider UNII ChEBI ChEMBL CompTox Dashboard (EPA ) Formula C 27 H 26 N 2 O 3 Molar mass −1 3D model (JSmol ) show CC1=C(C2=C3N1[C@@H](COC3=CC=C2)CN4CCOCC4)C(=O)C5=CC=CC6=CC=CC=C65
show InChI=1S/C27H26N2O3/c1-18-25(27(30)22-9-4-7-19-6-2-3-8-21(19)22)23-10-5-11-24-26(23)29(18)20(17-32-24)16-28-12-14-31-15-13-28/h2-11,20H,12-17H2,1H3/t20-/m1/s1
N Key:HQVHOQAKMCMIIM-HXUWFJFHSA-N
N N Y (what is this?)
Pancreatic stellate cells . The cells in the lower frame are under the action of WIN 55,212-2. They are thought to assume a more "
quiescent " phenotype. From Michalski et al., 2008.
[2] WIN 55,212-2 is a chemical described as an aminoalkylindole derivative, which produces effects similar to those of cannabinoids such as tetrahydrocannabinol (THC) but has an entirely different chemical structure .[3] [4] [5]
WIN 55,212-2 is a potent cannabinoid receptor agonist [6] [7] [8] p42 and p44 MAP kinase via receptor-mediated signaling.[9]
At 5 μM WIN 55,212-2 inhibits ATP production in sperm in a CB1 receptor -dependent fashion.[10]
WIN 55,212-2, along with HU-210 and JWH-133 , may prevent the inflammation caused by amyloid beta proteins involved in Alzheimer's disease , in addition to preventing cognitive impairment and loss of neuronal markers . This anti-inflammatory action is induced through agonist action at cannabinoid receptors , which prevents microglial activation that elicits the inflammation.
WIN 55,212-2 is a full agonist at the CB1 cannabinoid receptor (K i THC (K i = 41 nM) for this receptor.[11] PPARα and PPARγ nuclear receptors.[12]
WIN 55,212-2 reduces voluntary wheel running in laboratory mice, but with effects that depend on both genetic background and sex.[13]
In the United States, all CB1 receptor agonists of the 3-(1-naphthoyl)indole class such as WIN 55,212-2 are Schedule I Controlled Substances .[14] [15]
See also [ ] WIN 48,098 (Pravadoline)WIN 54,461 (6-Bromopravadoline)WIN 55,225 (JWH-200)WIN 56,098 References [ ]
^ "Controlled Drugs and Substance Act - Schedule II" . Justice Laws Website . Government of Canada.^ Michalski CW, Maier M, Erkan M, Sauliunaite D, Bergmann F, Pacher P, et al. (February 2008). Gluud C (ed.). "Cannabinoids reduce markers of inflammation and fibrosis in pancreatic stellate cells" . PLOS ONE . 3 (2): e1701. Bibcode :2008PLoSO...3.1701M . doi :10.1371/journal.pone.0001701 . PMC 2253501 PMID 18301776 . ^ Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR (1992). "Aminoalkylindole Analogs: Cannabimimetic Activity of a Class of Compounds Structurally Distinct from Δ9 -Tetrahydrocannabinol". Journal of Pharmacology and Experimental Therapeutics . 263 (3): 1118–1126. PMID 1335057 . ^ Ferraro L, Tomasini MC, Gessa GL, Bebe BW, Tanganelli S, Antonelli T (August 2001). "The cannabinoid receptor agonist WIN 55,212-2 regulates glutamate transmission in rat cerebral cortex: an in vivo and in vitro study" . Cerebral Cortex . 11 (8): 728–33. doi :10.1093/cercor/11.8.728 PMID 11459762 . ^ Zhang Q, Ma P, Iszard M, Cole RB, Wang W, Wang G (October 2002). "In vitro metabolism of R(+)-[2,3-dihydro-5-methyl-3-[(morpholinyl)methyl]pyrrolo [1,2,3-de]1,4-benzoxazinyl]-(1-naphthalenyl) methanone mesylate, a cannabinoid receptor agonist". Drug Metabolism and Disposition . 30 (10): 1077–86. doi :10.1124/dmd.30.10.1077 . PMID 12228183 . ^ Felder CC, Joyce KE, Briley EM, Mansouri J, Mackie K, Blond O, et al. (September 1995). "Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors". Molecular Pharmacology . 48 (3): 443–50. PMID 7565624 . ^ Meng ID, Manning BH, Martin WJ, Fields HL (September 1998). "An analgesia circuit activated by cannabinoids". Nature . 395 (6700): 381–3. Bibcode :1998Natur.395..381M . doi :10.1038/26481 . PMID 9759727 . S2CID 1619608 . ^ Herzberg U, Eliav E, Bennett GJ, Kopin IJ (January 1997). "The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain". Neuroscience Letters . 221 (2–3): 157–60. doi :10.1016/S0304-3940(96)13308-5 . PMID 9121688 . S2CID 33643599 . ^ Bouaboula M, Poinot-Chazel C, Bourrié B, Canat X, Calandra B, Rinaldi-Carmona M, et al. (December 1995). "Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1" . The Biochemical Journal . 312 ( Pt 2) (Pt 2): 637–41. doi :10.1042/bj3120637 . PMC 1136308 PMID 8526880 . ^ Morgan DJ, Muller CH, Murataeva NA, Davis BJ, Mackie K (April 2012). "Δ9-Tetrahydrocannabinol (Δ9-THC) attenuates mouse sperm motility and male fecundity" . British Journal of Pharmacology . 165 (8): 2575–83. doi :10.1111/j.1476-5381.2011.01506.x . PMC 3423255 PMID 21615727 . ^ Kuster JE, Stevenson JI, Ward SJ, D'Ambra TE, Haycock DA (March 1993). "Aminoalkylindole binding in rat cerebellum: selective displacement by natural and synthetic cannabinoids". The Journal of Pharmacology and Experimental Therapeutics . 264 (3): 1352–63. PMID 8450470 . ^ O'Sullivan SE (June 2016). "An update on PPAR activation by cannabinoids" . British Journal of Pharmacology . 173 (12): 1899–910. doi :10.1111/bph.13497 . PMC 4882496 PMID 27077495 . ^ Keeney BK, Meek TH, Middleton KM, Holness LF, Garland T (June 2012). "Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectively bred for high voluntary wheel-running behavior". Pharmacology, Biochemistry, and Behavior . 101 (4): 528–37. doi :10.1016/j.pbb.2012.02.017 . PMID 22405775 . S2CID 25174208 . ^ 21 U.S.C. § 812 Schedules of controlled substances ^ "The Misuse of Drugs Act 1971 (Amendment) Order 2013" . legislation.gov.uk .
Further reading [ ]
Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM (July 2006). "The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin" . Proceedings of the National Academy of Sciences of the United States of America . 103 (30): 11393–8. Bibcode :2006PNAS..10311393P . doi :10.1073/pnas.0603861103 . PMC 1544096 PMID 16849427 . Ramírez BG, Blázquez C, Gómez del Pulgar T, Guzmán M, de Ceballos ML (February 2005). "Prevention of Alzheimer's disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation" . The Journal of Neuroscience . 25 (8): 1904–13. doi :10.1523/JNEUROSCI.4540-04.2005 . PMC 6726060 PMID 15728830 .
External links [ ] show Hallucinogens
Psychedelics (5-HT2A
Benzofurans
2C-B-FLY 2CBFly-NBOMe 5-MeO-BFE 5-MeO-DiBF Bromo-DragonFLY F-2 F-22 TFMFly Lyserg‐
1B-LSD 1cP-LSD 1P-ETH-LAD 1P-LSD 1V-LSD 2-Butyllysergamide 3-Pentyllysergamide AL-LAD ALD-52 BU-LAD Diallyllysergamide Dimethyllysergamide ECPLA Ergometrine ETH-LAD IP-LAD LAE-32 LPD-824 LSA LSD LSD-Pip LSH LSM-775 LSZ Methylergometrine MIPLA Methysergide MLD-41 PARGY-LAD PRO-LAD Phenethyl‐
Piperazines Tryptamines
alpha -alkyltryptamines
4,5-DHP-α-MT 5-MeO-α-ET 5-MeO-α-MT α-ET α-MT x -DALT
(Daltacetin) 4-AcO-DALT 5-MeO-DALT DALT x -DET
(Ethacetin) 4-AcO-DET (Ethocin) 4-HO-DET 5-MeO-DET (T-9) DET (Ethocybin) 4-PO-DET x -DiPT
(Ipracetin) 4-AcO-DiPT (Iprocin) 4-HO-DiPT 5-MeO-DiPT DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4-Propionyloxy-DMT 5-Bromo-DMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT
(Deprocin) 4-HO-DPT 5-MeO-DPT (The Light) DPT Ibogaine-related
18-MAC 18-MC Coronaridine Ibogaine Ibogamine ME-18-MC Noribogaine Tabernanthine Voacangine x -MET
(Metocin) 4-HO-MET (Metocetin) 4-AcO-MET 5-MeO-MET MET x -MiPT
(Mipracetin) 4-AcO-MiPT (Miprocin) 4-HO-MiPT 5-Me-MiPT (Moxy) 5-MeO-MiPT MiPT Others
4-HO-DBT 4-HO-EPT 4-HO-McPT (Lucigenol) 4-HO-MPMI (Meprocin) 4-HO-MPT 5-MeO-EiPT 5-MeO-MALT 5-MeO-MPMI Aeruginascin Baeocystin DBT EiPT EPT MPT PiPT
Others
AL-38022A ALPHA Dimemebfe Efavirenz Glaucine Lorcaserin M-ALPHA RH-34 Also empathogens in general (e. g.: 5-APB , 5-MAPB , 6-APB and other substituted benzofurans ).
Dissociatives (NMDAR antagonists )
Arylcyclo‐
Ketamine-related
2-Fluorodeschloroketamine Arketamine ((R)-ketamine)Deschloroketamine Ethketamine (N-Ethylnorketamine)Esketamine ((S)-ketamine)Ketamine Methoxetamine Methoxmetamine Methoxyketamine Norketamine Tiletamine PCP-related Others
Diarylethylamines
Diphenidine Ephenidine Fluorolintane Methoxphenidine Morphinans
Dextrallorphan Dextromethorphan Dextrorphan Racemethorphan Racemorphan Others
2-EMSB 2-MDP 8A-PDHQ Aptiganel Budipine Delucemine Dexoxadrol Dizocilpine Etoxadrol Herkinorin Ibogaine Midafotel NEFA Neramexane Nitrous oxide Noribogaine Perzinfotel RB-64 Remacemide Selfotel Xenon
Deliriants (mAChR antagonists )
Atropine Benactyzine Benzatropine Benzydamine Biperiden Brompheniramine BZ CAR-226,086 CAR-301,060 CAR-302,196 CAR-302,282 CAR-302,668 Chloropyramine Chlorphenamine Clemastine CS-27349 Cyclizine Cyproheptadine Dicycloverine Dimenhydrinate Diphenhydramine Ditran Doxylamine EA-3167 EA-3443 EA-3580 EA-3834 Elemicin Flavoxate Hyoscyamine JB-318 JB-336 Meclozine Mepyramine Myristicin Orphenadrine Oxybutynin Pheniramine Phenyltoloxamine Procyclidine Promethazine Scopolamine Tolterodine Trihexyphenidyl Tripelennamine Triprolidine WIN-2299 Others
show Receptor (ligands )
CB1
Agonists (abridged; see here for more) : 2-AG 2-AGE (noladin ether) 11-Hydroxy-THC α-Amyrin · β-Amyrin AB-CHMINACA AM-1220 AM-1221 AM-1235 AM-2201 AM-2232 Anandamide AZ-11713908 Cannabinol CB-13 CP 47,497 CP 55,940 Dimethylheptylpyran DEA ECG EGCG Epicatechin Gallocatechol (gallocatechin) Honokiol HU-210 JWH-007 JWH-015 JWH-018 JWH-073 Kavain L-759,633 Levonantradol Menabitan Nabilone Nabitan NADA O-1812 Oleamide Pravadoline Serinolamide A THC (dronabinol) UR-144 WIN 55,212-2 Yangonin Antagonists: AM-251 AM-6545 Cannabidiol Cannabigerol Drinabant Falcarinol (carotatoxin) Hemopressin Ibipinabant LY-320,135 MK-9470 NESS-0327 O-2050 Otenabant PF-514273 PipISB Rimonabant Rosonabant Surinabant Taranabant THCV TM-38837 VCHSR Virodhamine CB2
Agonists: 2-AG 2-AGE (noladin ether) 3,3'-Diindolylmethane 4-O-Methylhonokiol α-Amyrin · β-Amyrin A-796,260 A-834,735 A-836,339 AM-1221 AM-1235 AM-1241 AM-2232 Anandamide AZ-11713908 Cannabinol Caryophyllene CB-13 CBS-0550 CP 55,940 GW-405,833 (L-768,242) GW-842,166X HU-308 JTE 7-31 JWH-007 JWH-015 JWH-018 JWH-133 L-759,633 L-759,656 Magnolol MDA-19 Nabitan NADA Olorinab (APD-371) PF-03550096 S-444,823 SER-601 Serinolamide A UR-144 Tedalinab THC (dronabinol) THCV Virodhamine Antagonists: 4-O-Methylhonokiol AM-630 BML-190 Cannabidiol Honokiol JTE-907 SR-144,528 WIN 54,461 WIN 56,098 NAGly GPR18 )
Agonists: Abnormal cannabidiol ACPA AM251 Anandamide Cannabidiol NADGly THC (dronabinol) O-1602 GPR55
Agonists: 2-AGE (noladin ether) Abnormal cannabidiol AM-251 CP 55,940 Lysophosphatidylinositol O-1602 Oleoylethanolamide Palmitoylethanolamide THC (dronabinol) GPR119 Unsorted
Transporter (modulators )
eCBTs
Inhibitors: AM-404 Arachidonoyl serotonin Cannabidiol Guineensine LY-2183240 Paracetamol (acetaminophen) URB-597 VDM-11
Enzyme (modulators )
FAAH MAGL
Inhibitors: IDFP JZL-184 JZL-195 MAFP URB-602 ABHD6 ABHD12
Inhibitors: Betulinic acid Maslinic acid MAFP Oleanolic acid Orlistat (tetrahydrolipstatin) Ursolic acid
Others
Precursors: Phosphatidylethanolamine NAPE Diacylglycerol Others: (directly potentiates activity of 2-AG at CB1 receptor) (FAAH-like anandamide transporter inhibitor)
See also
Receptor/signaling modulators Cannabinoids (cannabinoids by structure)
show TRPA
Activators
4-Hydroxynonenal 4-Oxo-2-nonenal 4,5-EET 12S-HpETE 15-Deoxy-Δ12,14 -prostaglandin J2 α-Sanshool (ginger , Sichuan and melegueta peppers )Acrolein Allicin (garlic )Allyl isothiocyanate (mustard , radish , horseradish , wasabi )AM404 ASP-7663 Bradykinin Cannabichromene (cannabis )Cannabidiol (cannabis )Cannabigerol (cannabis )Cinnamaldehyde (cinnamon )CR gas (dibenzoxazepine; DBO) CS gas (2-chlorobenzal malononitrile) Cuminaldehyde (cumin )Curcumin (turmeric ) (celery )
Diallyl disulfide Dicentrine (Lindera Formalin Gingerols (ginger )Hepoxilin A3 Hepoxilin B3 Hydrogen peroxide Icilin Isothiocyanate JT-010 (celery , Angelica acutiloba
Linalool (Sichuan pepper , thyme )Methylglyoxal Methyl salicylate (wintergreen )N-Methylmaleimide Nicotine (tobacco )Oleocanthal (olive oil )Paclitaxel (Pacific yew )Paracetamol (acetaminophen) PF-4840154 Phenacyl chloride Polygodial (Dorrigo pepper )Shogaols (ginger , Sichuan and melegueta peppers )Tear gases Tetrahydrocannabinol (cannabis )Tetrahydrocannabiorcol Thiopropanal S-oxide (onion )Umbellulone (Umbellularia californica )WIN 55,212-2 Blockers
TRPC
Activators
Adhyperforin (St John's wort )Diacyl glycerol GSK1702934A Hyperforin (St John's wort )Substance P Blockers
DHEA-S Flufenamic acid GSK417651A Meclofenamic acid N-(p-Amylcinnamoyl)anthranilic acid Niflumic acid Pregnenolone sulfate Progesterone Tolfenamic acid
TRPM
Activators
ADP-ribose Calcium (intracellular)CIM-0216 Cold Cooling Agent 10 Eucalyptol (eucalyptus )Geraniol Hydroxycitronellal Icilin Linalool Menthol (mint )Pregnenolone sulfate (Ruta graveolens
Steviol glycosides (e.g., stevioside ) (Stevia rebaudiana Sweet tastants (e.g., glucose , fructose , sucrose ; indirectly)
WS-12 Blockers
AMG-333 Capsazepine Clotrimazole Flufenamic acid Meclofenamic acid Mefenamic acid N-(p-Amylcinnamoyl)anthranilic acid Nicotine (tobacco )Niflumic acid Ononetin PF-05105679 RQ-00203078 Ruthenium red (Ruta graveolens
Tolfenamic acid TPPO TRPM4-IN-5
TRPML
TRPP
Activators
Triptolide (Tripterygium wilfordii Blockers
TRPV
Activators
2-APB 5',6'-EET 9-HODE 9-oxoODE 12S-HETE 12S-HpETE 13-HODE 13-oxoODE 20-HETE α-Sanshool (ginger , Sichuan and melegueta peppers )Allicin (garlic )AM404 Anandamide (Andrographis paniculata
Camphor (camphor laurel , rosemary , camphorweed , African blue basil , camphor basil )Cannabidiol (cannabis )Cannabidivarin (cannabis )Capsaicin (chili pepper )Carvacrol (oregano , thyme , pepperwort , wild bergamot , others)DHEA Diacyl glycerol Dihydrocapsaicin (chili pepper )Estradiol Eugenol (basil , clove )Evodiamine (Euodia ruticarpa Gingerols (ginger )GSK1016790A Heat Hepoxilin A3 Hepoxilin B3 Homocapsaicin (chili pepper )Homodihydrocapsaicin (chili pepper )Incensole (incense )Lysophosphatidic acid Low pH (acidic conditions)
Menthol (mint )N-Arachidonoyl dopamine N-Oleoylethanolamide Nonivamide (PAVA) (PAVA spray )Nordihydrocapsaicin (chili pepper )Paclitaxel (Pacific yew )Paracetamol (acetaminophen) Phenylacetylrinvanil Phorbol esters (e.g., )Piperine (black pepper , long pepper )Polygodial (Dorrigo pepper )Probenecid Protons RhTx (Ruta graveolens
Resiniferatoxin (RTX) (Euphorbia resinifera /pooissonii Shogaols (ginger , Sichuan and melegueta peppers )Tetrahydrocannabivarin (cannabis )Thymol (thyme , oregano )Tinyatoxin (Euphorbia resinifera /pooissonii Tramadol Vanillin (vanilla )Zucapsaicin Blockers
α-Spinasterol (Vernonia tweediana AMG-517 AMG-9810 Cannabigerol (cannabis )Cannabigerolic acid (cannabis ) (cannabis )
Cannabinol (cannabis )Capsazepine DHEA DHEA-S Flufenamic acid GRC-6211 HC-067047 Lanthanum Mavatrep Meclofenamic acid N-(p-Amylcinnamoyl)anthranilic acid Niflumic acid Pregnenolone sulfate RN-9893 Ruthenium red SB-705498 Tolfenamic acid TRPV3-74a
See also: Receptor/signaling modulators • Ion channel modulators