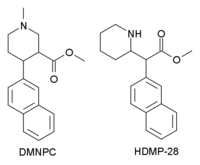
N ,O -Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate
methyl (3S ,4S )- 1-methyl- 4-(2-naphthyl)piperidine- 3-carboxylate
CAS Number PubChem CID CompTox Dashboard (EPA ) Formula C 18 H 21 N O 2 Molar mass −1 3D model (JSmol )
COC(=O)C1CN(C)CCC1c3ccc2ccccc2c3
N ,O -Dimethyl-4β-(2-naphthyl)piperidine-3β-carboxylateDMNPC ) is a piperidine based stimulant drug which is synthesised from arecoline . It is similar to nocaine in chemical structure, and has two and a half times more activity than cocaine as a dopamine reuptake inhibitor . However it is also a potent serotonin reuptake inhibitor , with similar affinity to fluoxetine .[1]
Ki Affinity of Piperidine Based MAT Inhibitors
∗∗
X
N
5HT
DA
NE
SS
p -VinylMe
138
131
175
p -Ethyl255
>1.7K
>1.1K
p -Allyl309
964
>1K
p -Ethynyl175
187
364
p -Phenyl62
173
203
β-Naphthyl
7.6
21
34
3R,4S
42
947
241
RR
192
87
27
3S,4R
12
271
38
H2 Cl
3.5
90
30
SS/RR
α-Naphthyl
Me
101
304
281
Clearly it is not just the SS enantiomer of the title compound that is an active MAT inhibitor.
Effect of N -demethylation [ ] For the SR enantiomer, increased DAT affinity is seen upon demethylation.
This is the same choice of isomer used in the production of Paxil .
See also: RTI-274
Simplification [ ] A substantially simpler method that ablates the carbomethoxy ester substituent can be found by D. Koch in Ex2: U.S. Patent 6,303,627
See also [ ] References [ ]
^ Tamiz AP, Zhang J, Flippen-Anderson JL, Zhang M, Johnson KM, Deschaux O, et al. (March 2000). "Further SAR Studies of Piperidine-Based Analogues of Cocaine. 2. Potent Dopamine and Serotonin Reuptake Inhibitors". Journal of Medicinal Chemistry . 43 (6): 1215–22. doi :10.1021/jm9905561 . PMID 10737754 .
Stimulants
Adamantanes
Adapromine Amantadine Bromantane Memantine Rimantadine Adenosine antagonists Alkylamines
Cyclopentamine Cypenamine Cyprodenate Heptaminol Isometheptene Levopropylhexedrine Methylhexaneamine Octodrine Propylhexedrine Tuaminoheptane Ampakines Arylcyclohexylamines Benzazepines
6-Br-APB SKF-77434 SKF-81297 SKF-82958 Cathinones Cholinergics Convulsants Eugeroics Oxazolines Phenethylamines Phenylmorpholines Piperazines
2C-B-BZP 3C-PEP BZP CM156 DBL-583 GBR-12783 GBR-12935 GBR-13069 GBR-13098 GBR-13119 MeOPP MBZP oMPP Vanoxerine Piperidines
1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine 2-Benzylpiperidine 2-Methyl-3-phenylpiperidine 3,4-Dichloromethylphenidate 4-Benzylpiperidine 4-Fluoromethylphenidate 4-Methylmethylphenidate Desoxypipradrol Difemetorex Diphenylpyraline Ethylnaphthidate Ethylphenidate Methylnaphthidate Isopropylphenidate JZ-IV-10 Methylphenidate (Dexmethylphenidate )Nocaine Phacetoperane Pipradrol Propylphenidate SCH-5472 Pyrrolidines Racetams
Oxiracetam Phenylpiracetam Phenylpiracetam hydrazide Tropanes Tryptamines Others ATC code : N06B
D1 -like
Agonists
Ergolines : Cabergoline CY-208,243 Dihydroergocryptine Lisuride Pergolide Terguride Phenethylamines : Deoxyepinephrine (N-methyldopamine, epinine) Dopexamine Etilevodopa Ibopamine L -DOPA (levodopa)Melevodopa L -PhenylalanineL -TyrosineOthers : A-68930 Apomorphine Nuciferine PF-6649751 Propylnorapomorphine Rotigotine SKF-89,145 Stepholidine Tavapadon Tetrahydropalmatine PAMs Antagonists
Typical antipsychotics : Butaclamol Chlorpromazine Chlorprothixene Flupentixol (flupenthixol) (+melitracen )Fluphenazine Loxapine Perphenazine (+amitriptyline )Thioridazine Thiothixene Trifluoperazine (+tranylcypromine )Zuclopenthixol Atypical antipsychotics : Asenapine Clorotepine Clotiapine Clozapine DHA-clozapine Fluperlapine Iloperidone Norclozapine Olanzapine (+fluoxetine )Paliperidone Quetiapine Risperidone Zicronapine Ziprasidone Zotepine Others : Ecopipam EEDQ Metitepine (methiothepin) Perlapine SCH-23390
D2 -like
Agonists
Adamantanes : Amantadine Memantine Rimantadine Aminotetralins : 5-OH-DPAT 7-OH-DPAT 8-OH-PBZI Rotigotine UH-232 Ergolines : Bromocriptine Cabergoline Chanoclavine Dihydroergocryptine Epicriptine Ergocornine Lergotrile Lisuride LSD Pergolide Terguride Phenethylamines : Deoxyepinephrine (N-methyldopamine, epinine) Dopexamine Etilevodopa Ibopamine L -DOPA (levodopa)L -PhenylalanineL -TyrosineMelevodopa Atypical antipsychotics : Alentemol (U-66444B) Aripiprazole (+sertraline )Aripiprazole lauroxil Bifeprunox Brexpiprazole Brilaroxazine Cariprazine F-15063 Lumateperone Norclozapine Others : 3-PPP A-412997 ABT-670 ABT-724 Adrafinil Aplindore Apomorphine Arketamine Armodafinil BP-897 Captodiame CP-226,269 Dizocilpine Esketamine Flibanserin Ketamine Mesulergine Modafinil OSU-6162 Pardoprunox PD-128,907 PD-168,077 PF-219,061 PF-592,379 Phencyclidine Piribedil Pramipexole Preclamol Propylnorapomorphine Pukateine Quinagolide Quinelorane Quinpirole RDS-127 Ro10-5824 Ropinirole Roxindole Salvinorin A SKF-83,959 Sumanirole Talipexole Umespirone WAY-100,635 Antagonists
Antiemetics/gastroprokinetics/sedatives : Aceprometazine AS-8112 Alimemazine Alizapride Benzquinamide Bromopride Clebopride Deudomperidone Domperidone Eticlopride Hydroxyzine Itopride Metoclopramide Metopimazine Promethazine Thiethylperazine Trimethobenzamide Antidepressants : Amoxapine Nefazodone Opipramol Propiomazine Trimipramine Others : 3-PPP Azapride Bromerguride Bromocriptine Buspirone Desmethoxyfallypride EEDQ F-15063 Fallypride Fananserin Fenfluramine Iodobenzamide L-741,626 L-745,870 Levofenfluramine Metergoline Metitepine (methiothepin) N-Methylspiperone Nafadotride Nuciferine PNU-99,194 Pridopidine Raclopride Sarizotan SB-277,011-A Sonepiprazole Spiroxatrine Stepholidine Terguride Tetrahydropalmatine Tiapride UH-232 Yohimbine
See also: Receptor/signaling modulators Adrenergics Serotonergics Monoamine reuptake inhibitors Monoamine releasing agents Monoamine metabolism modulators Monoamine neurotoxins