Phosphatidylcholine

Phosphatidylcholines (PC) are a class of phospholipids that incorporate choline as a headgroup. They are a major component of biological membranes and can be easily obtained from a variety of readily available sources, such as egg yolk or soybeans, from which they are mechanically or chemically extracted using hexane. They are also a member of the lecithin group of yellow-brownish fatty substances occurring in animal and plant tissues. Dipalmitoyl phosphatidylcholine (a.k.a. lecithin) is a major component of pulmonary surfactant and is often used in the L/S ratio to calculate fetal lung maturity. While phosphatidylcholines are found in all plant and animal cells, they are absent in the membranes of most bacteria,[1] including Escherichia coli.[2] Purified phosphatidylcholine is produced commercially.
The name lecithin was derived from Greek λέκιθος, lekithos 'egg yolk' by Theodore Nicolas Gobley, a French chemist and pharmacist of the mid-19th century, who applied it to the egg yolk phosphatidylcholine that he identified in 1847. Gobley eventually completely described his lecithin from chemical structural point of view, in 1874. Phosphatidylcholines are such a major component of lecithin that in some contexts the terms are sometimes used as synonyms. However, lecithin extracts consist of a mixture of phosphatidylcholine and other compounds. It is also used along with sodium taurocholate for simulating fed- and fasted-state biorelevant media in dissolution studies of highly lipophilic drugs.
Phosphatidylcholine is a major constituent of cell membranes and pulmonary surfactant, and is more commonly found in the exoplasmic or outer leaflet of a cell membrane. It is thought to be transported between membranes within the cell by phosphatidylcholine transfer protein (PCTP).[3]
Phosphatidylcholine also plays a role in membrane-mediated cell signaling and PCTP activation of other enzymes.[4]
Structure and physical properties[]

This phospholipid is composed of a choline head group and glycerophosphoric acid, with a variety of fatty acids. Usually, one is a saturated fatty acid (in the given figure, this is palmitic acid (hexadecanoic acid, H3C-(CH2)14-COOH); margaric acid (heptadecanoic acid, H3C-(CH2)15-COOH), identified by Gobley in egg yolk, also belong to that class); and the other is an unsaturated fatty acid (here oleic acid, or 9Z-octadecenoic acid, as in Gobley's original egg yolk lecithin). However, there are also examples of disaturated species. Animal lung phosphatidylcholine, for example, contains a high proportion of dipalmitoylphosphatidylcholine.[5]
Phospholipase D catalyzes the hydrolysis of phosphatidylcholine to form phosphatidic acid (PA), releasing the soluble choline headgroup into the cytosol.
Possible health benefits[]
Senescence[]
A 2009 systematic review of clinical trials in humans found that there was not enough evidence to support supplementation of lecithin or phosphatidylcholine in dementia. The study found that a moderate benefit could not be ruled out until further large scale studies were performed.[6]
Lipolysis[]
Though phosphatidylcholine has been studied as an alternative to liposuction, there are no peer-reviewed studies that have shown it to have comparable effects.[7][8] Injection of phosphatidylcholine in lipomas has been studied, though results have been mixed.[9][10]
Ulcerative colitis[]
Treatment of ulcerative colitis with oral intake of phosphatidylcholine has been shown to result in decreased disease activity.[11]
Possible health risks[]
A 2011 report linked the microbial catabolites of phosphatidylcholine with increased atherosclerosis in mice through the production of choline, trimethylamine oxide, and betaine.[12]
Biosynthesis[]
Although multiple pathways exist for the biosynthesis of phosphatidylcholine, the predominant route in eukaryotes involves condensation between diacylglycerol (DAG) and cytidine 5'-diphosphocholine (CDP-choline or citicoline). The conversion is mediated by the enzyme diacylglycerol cholinephosphotransferase. Another pathway, mainly operative in the liver involves methylation of phosphatidylethanolamine with S-adenosyl methionine (SAM) being the methyl group donor.[13]
See also[]
- CDP choline
- Lysophosphatidylcholine
- Phosphatidylserine
- Saturated fatty acid
- Unsaturated fatty acid
Additional images[]
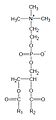
General structural formula of phosphatidylcholines

Membrane lipids
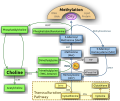
Choline metabolism

Phosphatidate

Choline
References[]
- ^ Jackowski S, Cronan JE, Rock CO (1991). "Chapter 2: Lipid metabolism in procaryotes". In Vance DE, Vance J (eds.). Biochemistry of Lipids, Lipoproteins and Membranes. Elsevier. pp. 80–81. ISBN 978-0-444-89321-5.
- ^ Chen F, Zhao Q, Cai X, Lv L, Lin W, Yu X, Li C, Li Y, Xiong M, Wang XG (November 2009). "Phosphatidylcholine in membrane of Escherichia coli changes bacterial antigenicity". Canadian Journal of Microbiology. 55 (11): 1328–34. doi:10.1139/w09-082. PMID 19940943.
- ^ Wirtz KW (July 1991). "Phospholipid transfer proteins". Annual Review of Biochemistry. 60 (13): 73–99. doi:10.1146/annurev.bi.60.070191.000445. PMID 1883207.
- ^ Kanno K, Wu MK, Agate DS, Fanelli BJ, Wagle N, Scapa EF, Ukomadu C, Cohen DE (October 2007). "Interacting proteins dictate function of the minimal START domain phosphatidylcholine transfer protein/StarD2". The Journal of Biological Chemistry. 282 (42): 30728–36. doi:10.1074/jbc.M703745200. PMID 17704541.
- ^ Christie WW. "Phosphatidylcholine and Related Lipids: Structure, Occurrence, Biochemistry and Analysis". Invergowrie, Dundee (DD2 5DA), Scotland.: James Hutton Institute. Archived from the original on 2014-12-11. Retrieved 2012-08-06.
{{cite web}}: CS1 maint: location (link) - ^ Higgins JP, Flicker L (21 January 2009). Higgins JP (ed.). "Lecithin for dementia and cognitive impairment". The Cochrane Database of Systematic Reviews. 4 (3): CD001015. doi:10.1002/14651858.CD001015. PMID 12917896.
- ^ Rotunda AM, Kolodney MS (April 2006). "Mesotherapy and phosphatidylcholine injections: historical clarification and review". Dermatologic Surgery. 32 (4): 465–80. CiteSeerX 10.1.1.506.2372. doi:10.1111/j.1524-4725.2006.32100.x. PMID 16681654. S2CID 9994696.
Recent laboratory investigations17 demonstrate that sodium deoxycholate, a bile salt also used as a laboratory detergent,102,103 was just as potent at causing adipocyte lysis and cell death as the complete phosphatidylcholine formula, which contains both phosphatidylcholine and deoxycholate (Figure 3). This bile salt is used to solubilize phosphatidylcholine by forming mixed micelles composed of phosphatidylcholine and deoxycholate.102,104 It is common practice to combine intravenous medications with bile salts to improve their water solubility.105,106 These findings suggest that sodium deoxycholate is the primary active ingredient in the phosphatidylchloline preparations.
- ^ Park SH, Kim DW, Lee MA, Yoo SC, Rhee SC, Koo SH, Seol GH, Cho EY (April 2008). "Effectiveness of mesotherapy on body contouring". Plastic and Reconstructive Surgery. 121 (4): 179e–85e. doi:10.1097/01.prs.0000304611.71480.0a. PMID 18349597. S2CID 22619355.
The author, when discussing phosphatidylcholine as a part of mesotherapy concludes: 'Although there is a preliminary report contradictory to this result, there was no body contouring observed in this study. There were no statistically significant changes in thigh girth, cross-sectional area, or laboratory values for the lipid profile except for a decrease in the triglyceride level in the blood, which might be an indirect effect of the method of aminophylline absorption into the systemic circulation.'
- ^ Amber KT, Ovadia S, Camacho I (June 2014). "Injection therapy for the management of superficial subcutaneous lipomas". The Journal of Clinical and Aesthetic Dermatology. 7 (6): 46–8. PMC 4086534. PMID 25013540.
- ^ Nanda S (May 2011). "Treatment of lipoma by injection lipolysis". Journal of Cutaneous and Aesthetic Surgery. 4 (2): 135–7. doi:10.4103/0974-2077.85040. PMC 3183720. PMID 21976907.
- ^ Kokkinidis DG, Bosdelekidou EE, Iliopoulou SM, Tassos AG, Texakalidis PT, Economopoulos KP, Kousoulis AA (September 2017). "Emerging treatments for ulcerative colitis: a systematic review". Scandinavian Journal of Gastroenterology. 52 (9): 923–931. doi:10.1080/00365521.2017.1326163. PMID 28503977. S2CID 4074211.
- ^ Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL (April 2011). "Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease". Nature. 472 (7341): 57–63. Bibcode:2011Natur.472...57W. doi:10.1038/nature09922. PMC 3086762. PMID 21475195.
- ^ Philip Y (2016-02-17). The membranes of cells (Third ed.). London. pp. 61–62. ISBN 9780128004869. OCLC 940961458.
External links[]
- Phosphatidylcholines at the US National Library of Medicine Medical Subject Headings (MeSH)
- [1]
- Phosphatidylcholines
- Cholinergics
- Phospholipids




