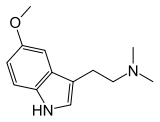
5-MeO-DMT
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Smoked, Insufflated, Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.012.558 |
| Chemical and physical data | |
| Formula | C13H18N2O |
| Molar mass | 218.300 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| | |
5-MeO-DMT (5-methoxy-N,N-dimethyltryptamine) or O-methyl-bufotenin is a psychedelic of the tryptamine class. It is found in a wide variety of plant species, and also is secreted by the glands of at least one toad species, the Sonoran Desert toad. Like its close relatives DMT and bufotenin (5-HO-DMT), it has been used as an entheogen in South America.[1] Slang terms include Five-methoxy, The power, and Toad venom.[2]
Chemistry[]
5-MeO-DMT was first synthesized in 1936, and in 1959 it was isolated as one of the psychoactive ingredients of Anadenanthera peregrina seeds used in preparing Yopo snuff. It was once believed to be a major component of the psychoactive effects of the snuff, although this has recently been shown to be unlikely, due to the limited or sometimes even non-existent quantity contained within the seeds, which instead achieve their psychoactivity from the O-demethylated metabolite of 5-MeO-DMT, bufotenin.[3][4] It is metabolized mainly by CYP2D6.[4]
Effects[]
Depending on whether smoked or insufflated, total duration of experience can last between 10 minutes for the former, or up to 2 hours for the latter. Effects vary and can range from radical perspective shifting and perception of new insights, euphoria, immersive experiences, dissociation and non-responsiveness, sensual/erotic enhancement, to dysphoria, fear, terror, and panic.[5]
Uses[]
It may have anti-anxiety and anti-depressant effects.[6][7]
Religious use[]
The Church of the Tree of Life, founded in California in 1971 by John Mann but now defunct, declared the use of 5-MeO-DMT to be a sacrament. From approximately 1971 to the late 1980s, 5-MeO-DMT was discreetly available to its members.[8][9] Between 1970 and 1990, smoking of 5-MeO-DMT on parsley was probably one of the two most common forms of ingestion in the United States.[9]
Pharmacology[]
5-MeO-DMT is a methoxylated derivative of DMT. Based on studies in rats, its pharmacological activity is believed to be mainly through serotonin receptors. Specifically, it shows high affinity for the 5-HT2 and 5-HT1A subtypes.[10] Additional mechanisms of action such as inhibition of monoamine reuptake may be involved.[11] A 2019 European study with 42 volunteers showed that a single inhalation produced sustained enhancement of satisfaction with life, and easing of anxiety, depression, and post-traumatic stress disorder (PTSD).[12]
In 2018, researchers also discovered that 5-MeO-DMT is a psychoplastogen, which refers to a compound capable of promoting rapid and sustained neural plasticity.[13]
Clinical Development[]
5-MeO-DMT is being developed and evaluated for potential therapeutic effects in patients suffering from (TRD).[14] Biopharmaceutical company GH Research Ireland Limited has sponsored a completed study in healthy volunteers and an ongoing study in TRD patients.[15]
Sources[]
This section is missing information about popularity of toad source; specific synthetic routes. (February 2021) |
5-MeO-DMT can be produced synthetically.[16][17]
| Family | Plants |
|---|---|
| Rutaceae | ,[18] Limonia acidissima,[19] [20] |
| Fabaceae | Anadenanthera peregrina,[21] Acacia auriculiformis,[21] Acacia Victoriae,[21] Desmodium gangeticum,[21] Lespedeza bicolor,[20][19] Mimosa pudica,[21] Mucuna pruriens,[19][20] Phyllodium pulchellum[19][20] |
| Poaceae | Phalaris tuberosa[21] |
| Malpighiaceae | Banisteriopsis caapi,[21] Diplopterys cabrerana[22] |
| Cactaceae | ,[19] Echinocereus triglochidiatus[19] |
| Myristicaceae | Horsfieldia superba,[19] ,[19] Osteophloeum platyspermum,[22] V. theiodora,[19] V. calophylla,[22] V. multinervia,[22] V. peruviana,[22] V. rufula,[22] V. venosa[22] |
| Family | Animals |
|---|---|
| Bufonidae | Colorado River toad (Bufo alvarius)[23][12][20] |
| Family | Fungi |
|---|---|
| Amanitaceae | Amanita citrina,[22] Amanita porphyria[22] |
Legal status[]
China[]
As of October 2015, 5-MeO-DMT is a controlled substance in China.[24]
Australia[]
As a structural analog of N,N-dimethyltryptamine (DMT), 5-MeO-DMT is a Schedule 9 prohibited substance under the Poisons Standard.[25]
Sweden[]
Sveriges riksdags health ministry Statens folkhälsoinstitut classified 5-MeO-DMT, listed as 5-metoxi-N,N-dimetyltryptamin (5-MeO-DMT) in their regulation SFS 2004:696, as "health hazard" under the act Lagen om förbud mot vissa hälsofarliga varor (translated Act on the Prohibition of Certain Goods Dangerous to Health) in October 2004, making it illegal to sell or possess.[26]
Germany[]
As of 2001 5-MeO-DMT is listed as a controlled substance. Attachement I BtMG. BGBl. I 2001, 1180 - 1186;
Turkey[]
5-MeO-DMT has been controlled in Turkey since December 2013.[27]
United States[]
5-MeO-DMT was made a Schedule I controlled substance in January 2011.[28]
See also[]
- 4-MeO-DMT
- 5-MeO-AMT
- 5-MeO-DIPT
- 5-EtO-DMT
- 5-MeO-MET
- Dimemebfe
- EMDT
- Hamilton's Pharmacopeia
- List of entheogens
References[]
- ^ Araújo AM, Carvalho F, Bastos M, Guedes de Pinho P, Carvalho M (August 2015). "The hallucinogenic world of tryptamines: an updated review". Archives of Toxicology. 89 (8): 1151–73. doi:10.1007/s00204-015-1513-x. PMID 25877327. S2CID 4825078.
- ^ "Ultimate Guide to 5-MeO-DMT - Experience, Benefits, & Side Effects". 29 June 2020.
- ^ Ott J (July–September 2001). "Pharmañopo-psychonautics: human intranasal, sublingual, intrarectal, pulmonary and oral pharmacology of bufotenine". Journal of Psychoactive Drugs. 33 (3): 273–81. doi:10.1080/02791072.2001.10400574. PMID 11718320. S2CID 5877023.
- ^ a b Shen HW, Jiang XL, Winter JC, Yu AM (October 2010). "Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions". Current Drug Metabolism. 11 (8): 659–66. doi:10.2174/138920010794233495. PMC 3028383. PMID 20942780.
- ^ "5-MeO-DMT Effects by Erowid". Erowid.org. Retrieved 2021-07-30.
- ^ Davis, Alan K.; So, Sara; Lancelotta, Rafael; Barsuglia, Joseph P.; Griffiths, Roland R. (March 2019). "5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety". The American Journal of Drug and Alcohol Abuse. 45 (2): 161–169. doi:10.1080/00952990.2018.1545024. PMC 6430661. PMID 30822141.
- ^ Davis, Alan K; Barsuglia, Joseph P; Lancelotta, Rafael; Grant, Robert M; Renn, Elise (30 April 2018). "The epidemiology of 5-methoxy-N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption". Journal of Psychopharmacology. 32 (7): 779–792. doi:10.1177/0269881118769063. PMC 6248886. PMID 29708042.
- ^ John Mann; Adam Gottlieb (2015) [First published 1970]. "back cover". The Book of Sacraments: Ritual Use of Magical Plants. Ronin Publishing. ISBN 978-1-57951-210-1.
- ^ a b "5-MeO-DMT Timeline". Erowid.
- ^ Krebs-Thomson K, Ruiz EM, Masten V, Buell M, Geyer MA (December 2006). "The roles of 5-HT1A and 5-HT2 receptors in the effects of 5-MeO-DMT on locomotor activity and prepulse inhibition in rats". Psychopharmacology. 189 (3): 319–29. doi:10.1007/s00213-006-0566-1. PMID 17013638. S2CID 23396616.
- ^ Nagai F, Nonaka R, Satoh Hisashi Kamimura K (March 2007). "The effects of non-medically used psychoactive drugs on monoamine neurotransmission in rat brain". European Journal of Pharmacology. 559 (2–3): 132–7. doi:10.1016/j.ejphar.2006.11.075. PMID 17223101.
- ^ a b Uthaug MV, Lancelotta R, van Oorsouw K, Kuypers KP, Mason N, Rak J, Šuláková A, Jurok R, Maryška M, Kuchař M, Páleníček T, Riba J, Ramaekers JG (September 2019). "A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms". Psychopharmacology. 236 (9): 2653–2666. doi:10.1007/s00213-019-05236-w. PMC 6695371. PMID 30982127.
- ^ Ly C, Greb AC, Cameron LP, Wong JM, Barragan EV, Wilson PC, Burbach KF, Soltanzadeh Zarandi S, Sood A, Paddy MR, Duim WC, Dennis MY, McAllister AK, Ori-McKenney KM, Gray JA, Olson DE (2018). "Psychedelics promote structural and functional neural plasticity". Cell Reports. 23 (11): 3170–3182. doi:10.1016/j.celrep.2018.05.022. PMC 6082376. PMID 29898390.
- ^ https://www.ghres.com
- ^ "Pipeline | GH Research".
- ^ Sherwood, Alexander M.; Claveau, Romain; Lancelotta, Rafael; Kaylo, Kristi W.; Lenoch, Kelsey (2020-12-02). "Synthesis and Characterization of 5-MeO-DMT Succinate for Clinical Use". ACS Omega. 5 (49): 32067–32075. doi:10.1021/acsomega.0c05099. ISSN 2470-1343. PMC 7745443. PMID 33344861.
- ^ Carpenter, David E. (2021-02-02). "Psychedelic Toads Pushed To The Limit, Conservationists Urge Synthetic 5-MeO-DMT Option". Forbes. Retrieved 2021-02-04.
- ^ Uthaug MV, Lancelotta R, Szabo A, Davis AK, Riba J, Ramaekers JG (March 2020). "Prospective examination of synthetic 5-methoxy-N,N-dimethyltryptamine inhalation: effects on salivary IL-6, cortisol levels, affect, and non-judgment". Psychopharmacology. 237 (3): 773–785. doi:10.1007/s00213-019-05414-w. PMC 7036074. PMID 31822925.
- ^ a b c d e f g h i "tryptamines: fungi". bluezoo.org.
- ^ a b c d e "Erowid Psychoactive Vaults: Tryptamine FAQ". www.erowid.org.
- ^ a b c d e f g "Some simple tryptomines" (PDF). troutsnotes.com. Retrieved 2020-07-04.
- ^ a b c d e f g h i Khan, JaVed I.; Kennedy, Thomas J.; Christian Jr, Donnell R. (2011). Basic Principles of Forensic Chemistry. Springer Science & Business Media. p. 196. ISBN 978-1-934115-06-0.
- ^ Carpenter DE. "5-MeO-DMT: The 20-Minute Psychoactive Toad Experience That's Transforming Lives". Forbes.
- ^ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Archived from the original on 1 October 2015. Retrieved 1 October 2015.
- ^ "Poisons Standard July 2016". Federal Register of Legislation.
- ^ "Förordning om ändring i förordningen (1999:58) om förbud mot vissa hälsofarliga varor" (PDF). Svensk författningssamling (in Swedish). 7 September 2004.
- ^ "Turkish Law" (PDF). Resmi Gazete. 16 December 2013.
- ^ Drug Enforcement Administration (DEA), Department of Justice (December 2010). "Schedules of controlled substances: placement of 5-methoxy-N,N-dimethyltryptamine into Schedule I of the Controlled Substances Act. Final rule" (PDF). Federal Register. 75 (243): 79296–300. PMID 21171485.
External links[]
- Ayahuasca
- Entheogens
- Psychedelic tryptamines
- Tryptamine alkaloids
- Indole ethers at the benzene ring
- Serotonin receptor agonists
- Psychedelic drugs
- Designer drugs
- Dimethylamino compounds