Norcocaine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.161.803 |
| Chemical and physical data | |
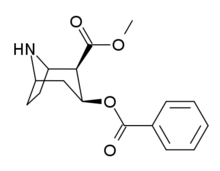
| Formula | C16H19NO4 |
| Molar mass | 289.331 g·mol−1 |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Norcocaine is a minor metabolite of cocaine. It is the only confirmed pharmacologically active metabolite of cocaine,[1] although salicylmethylecgonine is also speculated to be an active metabolite. The local anesthetic potential of norcocaine has been shown to be higher than that of cocaine,[2][3] however cocaine continues to be more widely used. Norcocaine used for research purposes is typically synthesized from cocaine. Several methods for the synthesis have been described.[4][5]
Legal status[]
The legal status of norcocaine is somewhat ambiguous. The US DEA does not list norcocaine as a controlled substance.[6] However, some suppliers of norcocaine, like Sigma-Aldrich, consider the drug to be a Schedule II drug (same as cocaine) for the purpose of their own sales.[7]
Toxicity[]
The LD50 of norcocaine has been studied in mice. When administered by the intraperitoneal route the LD50 in mice was 40 mg/kg.[8]
Role in hair drug testing controversy[]
Some researchers have suggested that hair drug testing for cocaine use should include testing for metabolites like norcocaine.[9] The basis for this suggestion is the potential for external contamination of hair during testing. There is considerable debate about whether current means of washing hair samples are sufficient for removing external contamination. Some researchers state the methods are sufficient,[10][11] while others state the residual contamination may result in a false positive test.[9][12][13][14] Metabolites of cocaine, like norcocaine, in addition to cocaine, should be present in samples from drug users. Authors have stated that the metabolites should be present in any samples declared positive.[9] Issues arise because the metabolites are present in only low concentrations. If the metabolites are present, it is possible for them to be from other contamination.[15]
References[]
- ^ "Virtual Mass Spectrometry Laboratory: Cocaine in Hair". Archived from the original on 2007-09-01. Retrieved 2008-01-14.
- ^ Wang Q, Simpao A, Sun L, Falk JL, Lau CE (January 2001). "Contribution of the active metabolite, norcocaine, to cocaine's effects after intravenous and oral administration in rats: pharmacodynamics". Psychopharmacology. 153 (3): 341–52. doi:10.1007/s002130000568. PMID 11271407. S2CID 10708670.
- ^ Just WW, Hoyer J (January 1977). "The local anesthetic potency of norcocaine, a metabolite of cocaine". Experientia. Birkhäuser. 33 (1): 70–1. doi:10.1007/BF01936761. PMID 836425. S2CID 32483810.
- ^ Stenberg VI, Narain NK, Singh SP, Parmar SS (April 1976). "An improved synthesis of norcocaine". Journal of Heterocyclic Chemistry. 13 (2): 363–364. doi:10.1002/jhet.5570130231.
- ^ Lazer ES, Aggarwal ND, Hite GJ, Nieforth KA, Kelleher RT, Spealman RD, et al. (December 1978). "Synthesis and biological activity of cocaine analogs I: N-alkylated norcocaine derivatives". Journal of Pharmaceutical Sciences. 67 (12): 1656–8. doi:10.1002/jps.2600671204. PMID 102759.
- ^ "Controlled Substances" (PDF). dea.gov. United States Drug Enforcement Administration, United States Department of Justice. 9 September 2014. Retrieved 8 December 2014.
- ^ "Norcocaine". sigmaaldrich.com. Sigma-Aldrich Co. LLC. Retrieved 8 December 2014.
- ^ Evans MA, Morarity T (January 1980). "Analysis of cocaine and cocaine metabolites by high pressure liquid chromatography". Journal of Analytical Toxicology. 4 (1): 19–22. doi:10.1093/jat/4.1.19. PMID 6927046.
- ^ a b c Cone EJ, Yousefnejad D, Darwin WD, Maguire T (1991). "Testing human hair for drugs of abuse. II. Identification of unique cocaine metabolites in hair of drug abusers and evaluation of decontamination procedures". Journal of Analytical Toxicology. 15 (5): 250–5. doi:10.1093/jat/15.5.250. PMID 1960975.
- ^ Koren G, Klein J, Forman R, Graham K (July 1992). "Hair analysis of cocaine: differentiation between systemic exposure and external contamination". Journal of Clinical Pharmacology. 32 (7): 671–5. doi:10.1002/j.1552-4604.1992.tb05780.x. PMID 1640006. S2CID 39938511.
- ^ Baumgartner WA, Hill VA (1992). "Hair analysis for drugs of abuse: Decontamination issues". Recent Developments in Therapeutic Drug Monitoring and Clinical Toxicology.
- ^ Henderson GL, Harkey MR, Jones RT, Zhou C (23 September 1991). "Effect of External Contamination on the Analysis of Hair for Cocaine". Paper Presented at the Joint Meeting of Forensic Toxicologists and the Canadian Society of Forensic Scientists, Montreal, Quebec, Canada.
- ^ Welch MJ, Sniegoski LT, Allgood CC, Habram M (1993). "Hair analysis for drugs of abuse: evaluation of analytical methods, environmental issues, and development of reference materials". Journal of Analytical Toxicology. 17 (7): 389–98. doi:10.1093/jat/17.7.389. PMID 8309210.
- ^ Blank DL, Kidwell DA (December 1993). "External contamination of hair by cocaine: an issue in forensic interpretation". Forensic Science International. 63 (1–3): 145–56, discussion 157–60. doi:10.1016/0379-0738(93)90268-f. PMID 8138216.
- ^ Janzen K (1992). "Concerning norcocaine, ethylbenzoylecgonine, and the identification of cocaine use in human hair". Journal of Analytical Toxicology. 16 (6): 402. doi:10.1093/jat/16.6.402. PMID 1293409.
- Drugs not assigned an ATC code
- Cocaine
- Stimulants
- Tropanes
- Benzoate esters
- Recreational drug metabolites